FY2021
Professor Erik De Schutter
Computational Neuroscience Unit
Abstract
We use computational, data-driven methods to study how neurons and microcircuits in the brain operate. We are interested in how fundamental properties, such as a neuron’s nanoscale morphology and its excitability, interact with one another during common neural functions like information processing or learning. Most of our models concern the cerebellum as this brain structure has a relatively simple anatomy and the physiology of its main neurons has been studied extensively, allowing for detailed modeling at many different levels of complexity. More recently we have also built models of hippocampal synapses.
1. Staff
- Molecular modeling
- Iain Hepburn, Technical Staff
- Andrew Gallimore, Staff Scientist (till March 2022)
- Sarah Yukie Nagasawa, PhD Student
- Cellular modeling
- Sungho Hong, Group Leader
- Alexey Martyushev, Postdoctoral scholar
- Gabriela Capo Rangel, Postdoctoral scholar
- Audrey Denizot, Postdoctoral scholar/JSPS Research Fellow
- Network modeling
- Mizuki Kato, PhD Student
- Software development
- Weiliang Chen, Staff Scientist
- Jules Lallouette, Postdoctoral scholar
- Visiting Researcher
- Sergio Verduzco, Visiting Researcher
- Jihwan Myung, Taipei Medical University
- Samuel Melchior, EPFL
- Tristan Carel, EPFL
- Giacomo Castiglioni, EPFL
- Alessandro Cattabiani, EPFL
- Yunliang Zang, Brandeis University
- Visiting Research Student
- Taro Yasuhi,Yamaguchi University School of Medicine
- Haruki Shigeta, Tohoku University School of Medicine
- Rotation Students
- Hugo Musset
- Josefine Reuschenbach
- Raj Aswathy
- Ryo Nakatani
- Research Unit Administrator
- Chie Narai
2. Collaborations
- Theme: Spiking activity of monkey cerebellar neurons
- Type of collaboration: Scientific collaboration
- Researchers:
- Professor H.P. Thier, University of Tübingen, Germany
- Akshay Markanday, University of Tübingen, Germany
- Junya Inoue, University of Tübingen, Germany
- Peter Dicke, University of Tübingen, Germany
- Theme: Human Brain Project: simulator development
- Type of collaboration: Scientific collaboration
- Researchers:
- Prof. F. Schürmann, École Polytechnique Fédérale de Lausanne, Switzerland
- Dr. T. Carel, École Polytechnique Fédérale de Lausanne, Switzerland
- Dr. G. Castiglioni, École Polytechnique Fédérale de Lausanne, Switzerland
- Dr. A. Cattabiani, École Polytechnique Fédérale de Lausanne, Switzerland
- Theme: Molecular identification of cerebellar signaling pathways and cerebellar optogenetics
- Type of collaboration: Scientific collaboration
- Researchers:
- Professor K. Tanaka, Korea Institute for Science and Technology (KIST), Korea
- Professor K. Tanaka, Korea Institute for Science and Technology (KIST), Korea
- Theme: Quantitative molecular identification of hippocampal synapses
- Type of collaboration: Scientific collaboration
- Researchers:
- Prof. Dr. Silvio O. Rizzoli, Medical University Göttingen, Germany
- Theme: Cerebellar molecular layer interneurons
- Type of collaboration: Joint research
- Researchers:
- Professor Alain Marty, Université Paris 5 René Descartes, France
- Theme: Cerebellar anatomy and physiology
- Type of collaboration: Scientific collaboration
- Researchers:
- Professor C. De Zeeuw, Erasmus Medical Center, Rotterdam, The Netherlands
- Professor L.W.J. Bosman, Erasmus Medical Center, Rotterdam, The Netherlands
- Dr. M. Negrello, Erasmus Medical Center, Rotterdam, The Netherlands
- Theme: Purkinje cell morphology and physiology, modeling
- Type of collaboration: Scientific collaboration
- Researchers:
- Professor M. Häusser, University College London, United Kingdom
- Professor A. Roth, University College London, United Kingdom
- Dr. S. Dieudonné, Ecole Normale Supérieure, Paris, France
- Taro Yasuhi,Yamaguchi University School of Medicine
- Theme: Circadadian rhythm generation
- Type of collaboration: Scientific collaboration
- Researchers:
- Professor J. Myung, Taipei Medical University, Taiwan
3. Activities and Findings
3.1 Cellular mechanisms regulating firing and synaptic properties of neurons
Parallel fiber input evoked dendritic calcium spikes in Purkinje cells
Neuronal firing patterns are crucial to underpin circuit level behaviors. In cerebellar PCs, both spike rates and pauses are used for behavioral coding (Hong et al., eLife 2016), but the cellular mechanisms causing code transitions remain unknown. We use a well-validated PC model (Zang et al., Cell Reports 2018) to explore the coding strategy that individual PCs use to process parallel fiber (PF) inputs. It is known that dendritic Ca2+ spikes can be evoked with clustered PF synaptic input (Rancz and Häusser, PNAS 2010).
To mimic clustered PF input, 40 synapses were randomly distributed within a dendritic branch of the PC model firing spontaneously at 40 Hz (Zang and De Schutter, J. Neurosci. 2021). Once these synapses are simultaneously activated, dendritic spikes occur and are spatially constrained (Figure 1). Dendritic spikes are initiated at distal tips and propagate to proximal parts with reducing amplitudes, similar to experimantal data.
We analyzed spike properties and tested their thresholds, i.e., the number of synapses required for dendritic spike initiation.With weak input, dendritic response amplitudes linearly increase with the number of activated PF synapses (Figure 1H), suggesting a linear computation in PC dendrites (Walter and Khodakhah, J. Neurosci. 2006). Once a threshold of 35 synapses is reached, dendritic response amplitudes show a step increase and maintain a fixed value even with stronger input.
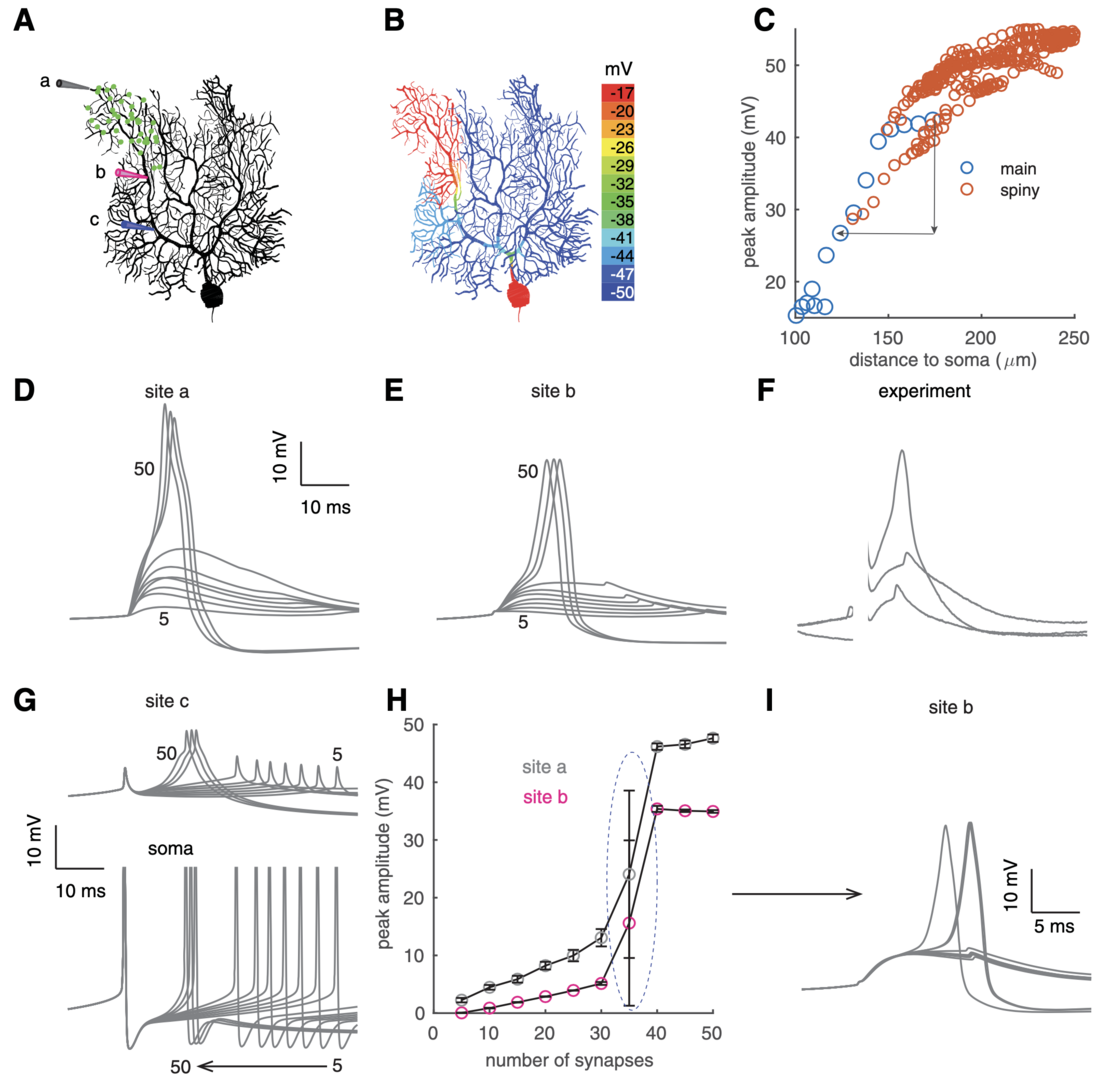
Figure 1: bimodal dendritic responses in individual PCs. (A) Clustered PF synapses within a branch (green dots). Three recording sites: a on the tip, b on the distal main dendrite, and c on the proximal main dendrite. (B) Color-coded voltage response peaks to clustered PF input. (C) Decayed spike propagation in somapetal direction. Red circles represent sites on spiny dendrites and blue circles represent sites on the main dendrite. Simulated membrane potentials at sites a and b with increasing PF synapses (from 5 to 50) are shown in D, E, respectively. (F) Experimentally measured dendritic responses with increasing stimulation intensity compared with site b in E (data shared by Ede Rancz and Michael Häusser; Rancz and Häusser, 2010). Initial parts of the traces were omitted because of stimulation artifacts. (G) Simulated membrane potentials at site c (above) and the soma (bottom, clipped) with increasing PF synapses (from 5 to 50). Note the backpropagated somatic Na+ spikes at site c. (H) Bimodal dendritic responses at sites a (gray circles) and b (pink circles) versus synchronously activated synapses. Under each condition (the same synaptic number), 10 trials were simulated with randomly distributed synapses. The spike threshold is circled. (I) Membrane potentials at site b with 35 PF synapses activated (n = 10). Some of the traces are overlapped, resulting in seemingly thicker lines (from Zang and De Schutter, 2021).
We find increasing input intensity shifts PCs from linear rate-coders to burst-pause timing-coders by triggering localized dendritic spikes. We elucidated spiking mechanisms (not shown). Spiking thresholds increase with inhibition (Figure 2).
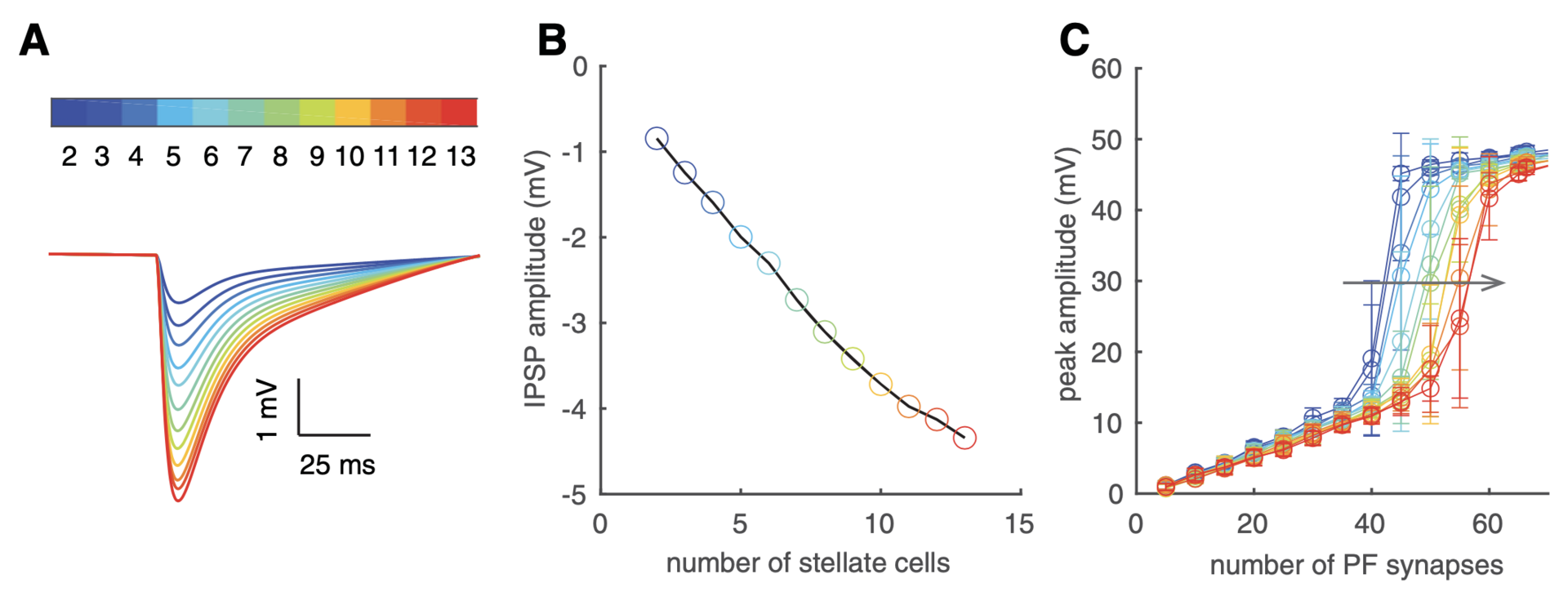
Figure 2: effectc of inhibition on dendritic spike thresholds. (A) Dendritic tip IPSPs with increased inhibition. (B) IPSP amplitudes lineraly increase with inhibiiton from more stellate cells. In A and B, the model was silenced by somatic holding current. (C) Inhibition right shifts dendritic spike thresholds (FFI delay is 1.4 ms). For A–C, color codes the number of activated stellate cells (each stellate cell form 16 synapses onto the PC), defined by the color bar in panel A (from Zang and De Schutter, 2021).
Both linear and burst-pause computations use individual branches as computational units, which challenges the traditional view of PCs as linear point neurons. How do PF-evoked dendritic spikes regulate somatic output? Example dendritic and somatic responses are shown in Figure 3. Below the dendritic spike threshold (50 synapses in branch 12), dendritic responses increase with PF input and elevate somatic spiking rate by triggering a burst clustered to PF input timing. Once dendritic spikes occur, the somatic burst is followed by a pause. When dendritic spikes are reliably triggered, the maximum spike rates during bursts and the durations of pauses are relatively invariant because of the all-or-none nature of dendritic spikes. These results suggest that PF dendritic spikes can trigger reliable burst-pause sequences.
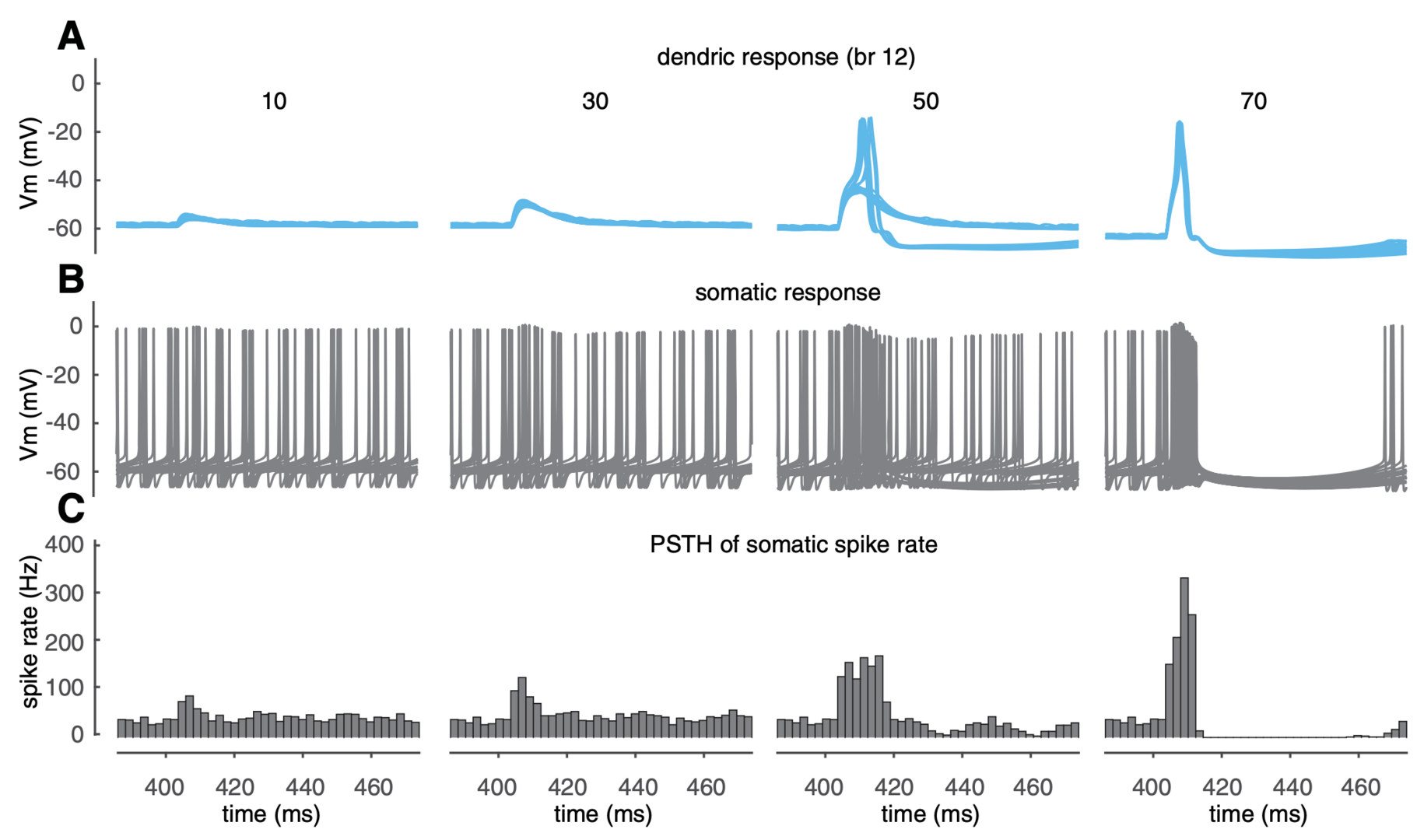
Figure 3: dendritic spikes trigger branch-specific somatic burst-pause sequences. (A, B) Membrane potentials at the dendritic tip and the soma, respectively (20 trials randomly selected from 500 simulation trials). (C) Peristimulus histograms of somatic spiking rate (bin size 2 ms, 500 simulation trials with randomly distributed PF synapses and disturbed somatic spike timing). From left to right in A– C, PF synapses increase from 10 to 70, and simulation results were obtained in branch 12 (from Zang and De Schutter, 2021).
Dendritic spike thresholds can be regulated by voltage state, compartmentalized channel modulation, between-branch interaction and synaptic inhibition to expand the dynamic range of linear computation or burst-pause computation. In addition, co-activated PF inputs between branches can modify somatic maximum spike rates and pause durations to make them carry analog signals. Our results provide new insights into the strategies used by individual neurons to expand their capacity of information processing.
4. Publications
4.1 Journals
- G.A. Ascoli, D.N. Kennedy and E. De Schutter: Farewell, Neuroinformatics! Neuroinformatics 19: 551-552.
- A. Denizot, C. Cali, H. Berry and E. De Schutter: Stochastic Spatially-Extended Simulations Predict the Effect of ER Distribution on Astrocytic Microdomain Ca2+ Activity. The Eight Annual ACM International Conference on Nanoscale Computing and Communication (NANOCOM ’21). doi.org/10.1145/3477206.3477456
4.2 Preprints
- S. Verduzco-Flores and E. De Schutter: Adaptive plasticity in the spinal cord can produce reaching from scratch and reproduces motor cortex directional tuning. Preprint at https://arxiv.org/abs/2109.07778
- A. Denizot, M. Arizono, U.V. Nägerl, H. Berry and E. De Schutter: Astrocyte nanoscale morphology controls Ca2+ signals at tripartite synapses.
Preprint at https://www.biorxiv.org/content/10.1101/2021.02.24.432635v1
4.3 Books and other one-time publications
Nothing to report
4.4 Oral and Poster Presentations
- A. Denizot, Computational approaches for simulating calcium signals in astrocytes: insights, limitations, challenges and perspectives, 1st Virtual Conference of the European Society for Neurochemistry “Future perspectives for European neurochemistry – a young scientist’sconference”, May 2021(Talk)
- A. Denizot, C. Cali, H. Berry, E. De Schutter, Elucidating the morphology of the endoplasmic reticulum in fine astrocyte branchlets and its effect on calcium signals., 1st Virtual Conference of theEuropean Society for Neurochemistry “Future perspectives for European neurochemistry– a young scientist’s conference”, May 2021(Poster)
- G. Capo Rangel and E. De Schutter, Branch Specific Dendritic Computation in a Purkinje Cell, CNS conference (30th Computational Neuroscience Meeting online), July 2021
- A. Denizot, Disentangling astrocytic calcium signals: insights from spatially-extended models, CNS conference (30th Computational Neuroscience Meeting online), July 2021.
- A. Denizot, M. Arizono, V. U. Nägerl, E. De Schutter, H. Berry., The nanoscale morphology of astrocyte branchlets governs local calcium activity, The 44th Annual Meeting of the Japan Neuroscience Society, Kobe Convention Center. July 2021.
- A. Denizot, C. Cali, H. Berry, E. De Schutter, Probing the localization of the endoplasmic reticulumin the gliapil and its effect on astrocytic calcium signals, XV European Meeting on Glial Cells in Health and Disease, GLIA 2021, July 2021(Poster)
- A. Denizot, Spatially-Extended Simulations Predict the Effect of ER Distribution onAstrocytic Microdomain Ca2+ Activity, 8th ACM International Conference on Nanoscale Computing and CommunicationVirtual Conference, Sept 2021. https://bit.ly/2YXwMKq
- A. Denizot, Decoding the astrocytic calcium code with computational approaches, 99th Annual Meeting of the Physiological Society of Japan, Sendai, March 2022.
- S. Hong, J. Kwon, J. Woo, S. Kim, E. De Schutter, and C. J. Lee, Computational modeling of age-dependent tonic inhibition in the cerebellar granule cells in a network context, 99th Annual Meeting of the Physiological Society of Japan, Sendai, March 2022 (Symposium Talk).
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
Nothing to report
7. Other
Nothing to report.



