Phosphoenolpyruvate (PEP) Treatment for Th17-Dependent Autoimmune Diseases (No. 0224)
|
|
<< Back to all technologies |
Summary
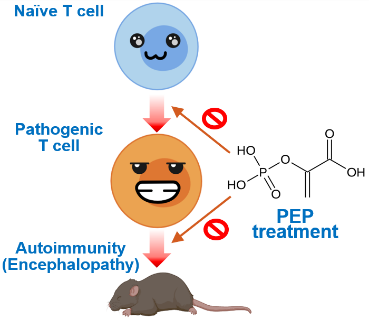
Autoimmune diseases affect nearly 4% of the world’s population. T helper 17 (Th17) cells play a critical role in autoimmune diseases such as multiple sclerosis and psoriasis. Although Th17-related cytokines or transcription factors are attractive therapeutic targets to combat autoimmune responses, treatments often also attack helpful, gut-resident Th17 cells, compromising gut homeostasis and host defense. A team of OIST researchers led by Prof. Hiroki Ishikawa has developed a novel, broad-spectrum therapeutic approach to suppress Th17-dependent autoimmune responses. The team discovered that phosphoenolpyruvate (PEP) inhibits JunB-dependent Th17 generation. As JunB is required for generation of autoimmune Th17 cells but not for gut-resident Th17 cells, PEP selectively targets autoimmune Th17 cells without triggering intestinal side effects.
Applications
- Drug development
- Multiple sclerosis and other autoimmune diseases
Advantages
- Selectively targets autoimmune Th17 cells without affecting gut Th17 cells
Technology
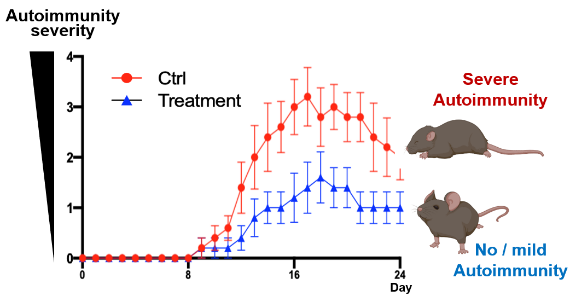
The researchers identified PEP, an intermediate metabolite of aerobic glycolysis, as a negative regulator of Th17 differentiation. Administering PEP was found to ameliorate Th17-dependent autoimmune encephalomyelitis in mice by inhibiting the expression of Th17 signature molecules, including IL-17A. PEP was found to bind to JunB, an AP-1 transcription factor that plays a key role in pathogenic Th17 differentiation, inhibiting DNA-binding of the JunB/BATF/IRF4 complex at the Il17a locus. PEP interacts with JunB proteins to mediate the interplay between aerobic glycolysis and Th17 differentiation, selectively targeting autoimmune cells.
Media Coverage and Presentations
CONTACT FOR MORE INFORMATION
![]() Graham Garner
Graham Garner
Technology Licensing Section
![]() tls@oist.jp
tls@oist.jp
![]() +81(0)98-966-8937
+81(0)98-966-8937