Novel Methods for Aryl- and Trifluoromethyl-Substituted Tertiary Alcohols Formation, Used for Numerous Applications (No. 0081)
|
|
|
<< Back to all technologies |
Summary
Molecules bearing aryl- and trifluoromethyl-substituted tertiary alcohol moieties are required as bioactive molecules, enantiomer-discriminating reagents, building blocks, and are specifically important in the pharmaceutical industry. However, despite their many applications, current reactions enabling to synthesize these molecules suffer from multiple setbacks, including complicated processes, long reaction times, and require either preactivation, or protection and deprotection steps. A novel method developed by a group of researchers led by Prof. Fujie Tanaka enables a fast, concise synthesis of these molecules. This process is based on aldol reactions of ketone donors with aryl trifluoromethyl ketone acceptors catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). It can be performed under mild conditions, requires short reaction times (1.5 h), and results with excellent yield (over 95%). Moreover, applying this method can result in perfect regioselectivity, expending product applications.
Applications
- Bioactive molecules
- Therapeutically active substances
- Enantiomer discriminating- and recognition-reagents
- Building blocks
- Complementary to other methods including organocatalytic reactions
Advantages
- Short reaction times (1.5 h)
- Excellent yield (>95%)
- Easily scaled up
- Mild conditions (25degC)
- Can enable enantiomerically pure forms
Technology
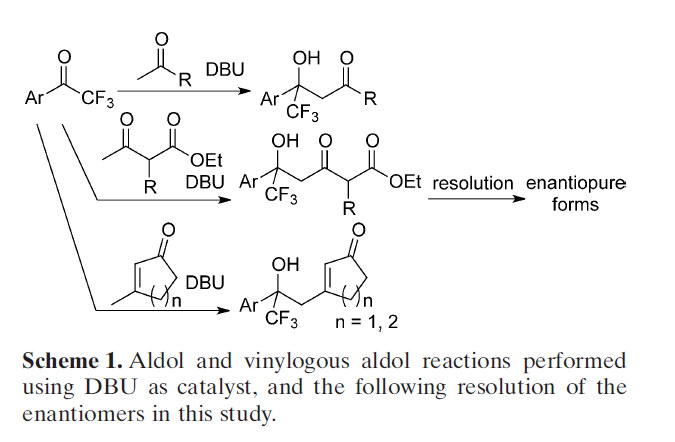
The researchers have developed aldol reactions of ketone donors with aryl trifluoromethyl ketone acceptors catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). These reactions enable generating molecules with tetrasubstituted carbon centers with trifluoromethyl-substituted alcohol moieties. Using these methods, C-C bonds of the aldol reactions are formed in perfect regioselectivities at the methyl group of the alkyl methyl ketones. The enantiomerically pure forms of the aldol products are obtained through resolution via the formation of enamines with a homochiral amine. Optimal results require only catalytic amounts of DBU, and reactions can be performed at room temperature for a short duration of 1.5 h.
Media Coverage and Presentations
CONTACT FOR MORE INFORMATION
![]() Paola Butler-Zanetti
Paola Butler-Zanetti
Technology Licensing Section
![]() tls@oist.jp
tls@oist.jp
![]() +81(0)98-966-8937
+81(0)98-966-8937