Non-Aqueous Buffer Compound (No. 0168)
|
|
|
<< Back to all technologies |
Summary
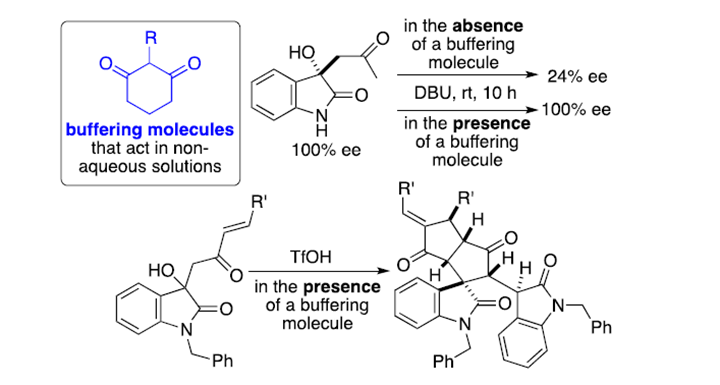
2-substituted-1,3-cyclohexanedione derivatives acting as buffering molecules for non-aqueous solutions.
Global bio pharma buffer market is expected to reach USD 980 Million by 2025, at a CAGR of 7.41%. Growing demand for vaccines, blood, blood components, allergenics, somatic cells, gene therapies, tissues, therapeutic recombinant protein, and living cells used in cell therapy are driving the global bio pharma buffer market. Maintaining product and reagent stability has long been an issue for the pharmaceutical and drug development industry. Buffers are used both for upstream and downstream development and production processes and a lack of a suitable buffer can result in unexpected manufacturing delays, product/reagent instability and major economic losses. However, no molecules that neutralize both acids and bases in organic solvents or that have buffering functions in non-aqueous solutions to maintain conditions suitable for chemical reactions have been commonly used. Here, we present a promising buffer developed by a group of researchers led by Prof. Fujie Tanaka. The suggested technology for non-aqueous solution media, proposes a simple solution to some of the production challenges with a potential to dramatically cut losses and increase derived financial benefits from existing processes.
Applications
- Drug Discovery
- Chemical Storage
Advantages
- Non-Aqueous Buffer Function
- Simple Production
- Chemical Reaction Control
Technology
Essentially, Prof. Tanaka has developed a molecule having a buffer function in a non-aqueous solution. Trace presence of acid or base often causes isomerization, decomposition, and/or racemization of molecules of interest. Addition of the developed buffering molecule or its resin-conjugated version in storage solutions of molecules of interest can prevent decomposition, isomerization, and/or racemization caused by both acids and bases.
Media Coverage and Presentations
CONTACT FOR MORE INFORMATION
![]() Graham Garner
Graham Garner
Technology Licensing Section
![]() tls@oist.jp
tls@oist.jp
![]() +81(0)98-966-8937
+81(0)98-966-8937