Synthesis of Novel Pharmaceutical Synthons (Spirooxindoles) (No. 0023)
|
|
|
<< Back to all technologies |
Summary
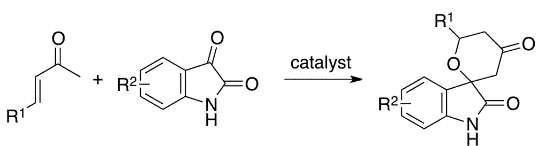
The chiral spirooxindole scaffold is present in a variety of bioactive alkaloids. Synthetic spirooxindoles have also been widely investigated for their anticancer, anti-inflammatory and analgesic activity, among others, with many drug candidates being tested in clinical trials with promising prospects. However, current metal-catalyzed synthetic methods involve complicated reaction conditions, whereas the aldol reaction of ketones to form quaternary carbon centers rarely affords high yields and stereoselectivity because of the reaction’s reversibility. A team of OIST researchers led by Prof. Fujie Tanaka has developed a novel synthetic approach to overcome the above problems and efficiently and stereoselectively synthesize novel spirooxindole derivatives from easily accessible starting materials under mild conditions.
Applications
- Bioactive compounds
- Drug development
Advantages
- High yield and stereoselectivity
- Mild conditions
- One-pot reaction
Technology
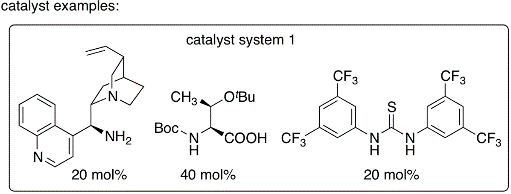
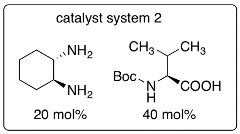
In this protocol, enones are used as enamine-forming reactants and the catalyst systems were developed to allow oxy-Michael addition after the aldol step. Because of the one pot aldol-oxy-Michael reaction (or formal hetero-Diels-Alder reaction), the stereoselectivity of the aldol step can be retained. This synthetic strategy is the first example of organocatalytic asymmetric aldol-oxy-Michael reactions and enables the synthesis of functionalized tetrahydropyran derivatives bearing quaternary carbon centers in high yields and with high diastereo- and enantioselectivities under mild conditions.
Media Coverage and Presentations
CONTACT FOR MORE INFORMATION
![]() Paola Butler-Zanetti
Paola Butler-Zanetti
Technology Licensing Section
![]() tls@oist.jp
tls@oist.jp
![]() +81(0)98-966-8937
+81(0)98-966-8937