Novel Method for Direct Synthesis of C-Glycosides from Unprotected 2-N-Acyl-Aldohexoses for Multiple Therapeutic Purposes (No. 0080)
|
|
|
<< Back to all technologies |
Summary
C-glycosides are highly stable bioactive molecules, widely known for their therapeutic activity. These molecules are found in nature and can be synthesized synthetically for multiple purposes: probes, building blocks and most importantly – for numerous therapeutic applications, such as anti-cancerous anti-diabetes, antiviral and anti-bacterial treatments. Despite the importance of these molecules, current methods for c-glycosides synthesis are relatively limited and do not provide access to important compounds with desired bioactive functions. Researchers led by Prof. Fujie Tanaka have developed a novel method for C-glycosides synthesis from unprotected carbohydrates and inactivated ketones via the C–C bond formation at the anomeric carbon of the carbohydrate. This method can provide C-glycoside derivatives that cannot be synthesized through previously reported methods. It is atom-economical, requires mild conditions, and results with products expressing high stereo-selectivity.
Applications
Generation of bioactive compounds for multiple purposes:
- C-glycosides, glycoconjugates, carbon-chain elongated carbohydrates and related compounds
- Molecules used as therapeutics, bioactive candidates, probes, synthons, and building blocks
Advantages
- Excellent stereoselectivity (>10:1)
- Mild reaction conditions (25 degC)
- Atom- and step-economical reactions
- Allows generation of products that were not demonstrated through other known methods
Technology
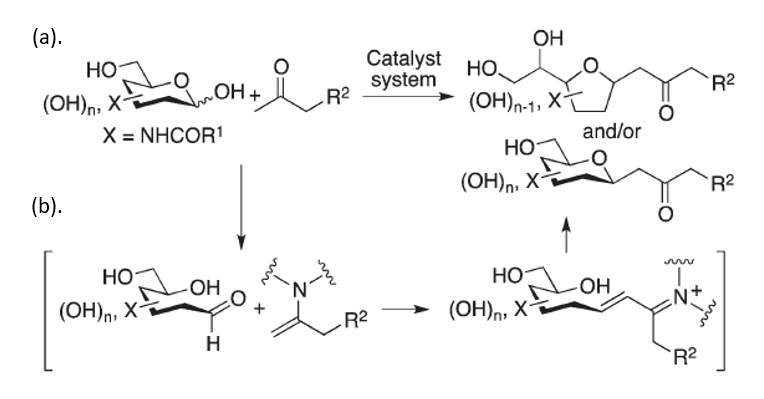
This novel strategy enables generation of C-glycosides from unprotected 2-N-acyl-aldohexoses through C–C bond formations at the anomeric centers of unprotected carbohydrates. In the first step, reactions are performed with 1,3-diketones via Knoevenagel condensation, followed by the elimination of the acyl group. Generation of the C-glycosides in the last step, involves aldol condensations of unprotected carbohydrates with simple ketones such as acetone, followed by oxa-Michael cyclization.
Media Coverage and Presentations
CONTACT FOR MORE INFORMATION
![]() Paola Butler-Zanetti
Paola Butler-Zanetti
Technology Licensing Section
![]() tls@oist.jp
tls@oist.jp
![]() +81(0)98-966-8937
+81(0)98-966-8937