FY2022 Annual Report
Chemistry and Chemical Bioengineering Unit
Professor Dr. Fujie Tanaka, Professor
Abstract
The ability to design and synthesize organic molecules constitutes the foundation that underlies basic research as well as applied science. The ability is also essential for the development of pharmaceuticals and biofunctional molecules. This unit develops efficient, concise, and safe chemical transformation methods and strategies for constructing small molecules bearing functional groups and/or chiral centers that are relevant to the creation of biofunctional molecules. We design and create small organic molecule catalyst systems for designed chemical transformations, and we develop reaction strategies that use small organic molecules as enzyme-like catalysts. With the use of organic molecules as catalysts, we minimize the need for protection and deprotection steps that are usually required for the synthesis of functionalized molecules. When a reaction method does not affect functional groups that are not at the reaction site, the reaction method can be used for the synthesis of a series of molecules bearing various functional groups. This means that a series of molecules of interest may be synthesized using the same method in a short route, providing advantages for the ynthesis of biofunctional candidate molecules. We also investigate the chemical bases of the reactions to understand the mechanisms of the catalysis and molecular interactions provided by organic molecules. By taking advantage of the use of features of our developing molecules, we also develop strategies and methods for conjugation of proteins and peptides with other molecules. The research undertaken by this unit advances the chemistry of catalysis and of molecular synthesis. The studies by this unit accelerate the creation of molecules used in biomedical research and contribute to the development of new therapeutics, therapeutic strategies, and diagnostic methods.
1. Staff
Dr. Ravindra D. Aher
Dr. Venkati Bethi
Dr. Santosh Chavan
Dr. Yuvraj Garg
Dr. Akash Jana
Dr. Maulik Mungalpara
Dr. Bapurao Dattatray Rupanawar
Dr. Muhammad Sohail
Dr. Prakash K. Warghude
Santanu Mondal, Graduate Student
James Osborne, Graduate Student
Kuan-Lin Chen, Graduate Student
Anjali Gupta, Graduate Student (Rotation)
Nivedha Velmurugan, Graduate Student (Rotation)
Tom Tassilo Wilfling, Graduate Student (Rotation)
Syeda Zahra, Research Intern
Shiho Chinen, Research Unit Administrator
2. Activities and Findings
2.1 Development of new chemical transformation methods and synthesis of functionalized molecules
We have been developing small organic molecule catalysts (organocatalysts) and organocatalytic molecular transformation methods useful for the synthesis of functionalized molecules under mild conditions in short routes. We also investigate the chemical bases of the catalyses and the chemical transformations to understand the mechanisms of the catalysis and molecular interactions provided by organic molecules to further the creation of useful molecules.
Traditional synthetic methods often require high or very low temperatures and/or absolute conditions. In addition, functional groups on substrate molecules must be protected prior to reactions. That is, depending on functional groups present in target molecules to be synthesized, synthetic routes, including protection and deprotection steps, have to be designed for each molecule. To concisely synthesize functionalized molecules, chemical transformation methods that are not affected by functional groups presenting in starting materials are needed. It is a great advantage when the same reaction method can be used for the synthesis of a series of molecules bearing various functional groups without the need of product-specific protection and deprotection steps. In addition, it is desired that such reactions can be performed under safe, mild, and environmentally benign conditions. We address these points in our research as we design and develop catalysts and chemical transformation methods. By using organic molecules as catalysts, we concisely synthesize novel functionalized molecules including those that are often difficult to synthesize by traditional synthetic strategies. Our investigations into the chemical basis of the developed catalysts and chemical transformation methods further the understanding of the chemistry of organic molecules and their reactions.
2.1.1. Direct enantioselective Mannich reactions of pyruvates catalyzed by amine-based catalyst systems: Control of reactions of pyruvates
Pyruvates or 2-oxopropanoates act as electrophiles and nucleophiles and are useful compounds for the synthesis of various molecules. In Nature, pyruvate is a precursor for amino acids, sugars, cofactors, and other biofunctional molecules. In enzyme-catalyzed reactions, pyruvates often serve as nucleophiles. In contrast, in the majority of non-enzymatic reactions of pyruvates, pyruvates serve as electrophiles due to the activated reactivity of the α-ketocarbonyl structure. Without the use of enzymes, the control of the nucleophilic reactivities of pyruvates, especially simple pyruvates such as ethyl pyruvate, is difficult. Reactions of pyruvates often results in the formation of self-aldol reaction products and cascade reaction products, in which pyruvates act as both nucleophiles and electrophiles. We have developed direct enantioselective Mannich reactions of pyruvates with cyclic imines catalyzed by an amine-based catalyst system, in which pyruvates act as nucleophiles without acting as electrophiles (Scheme 1) (Mondal, Aher, Bethi, Lin, Taniguchi, Monde, and Tanaka, Org. Lett. 2022, 24, 1853). The acidity and the structure of the acid used in the amine-based catalyst system influenced the yield and the enantioselectivity; the acid component of the amine-based catalyst system was key to obtain the desired Mannich products in high yields with high enantioselectivities. In addition, using the α-ketoester group of the products as the reaction site, α,γ-diamino acid derivatives and γ-amino α-hydroxyacid derivatives were readily synthesized.
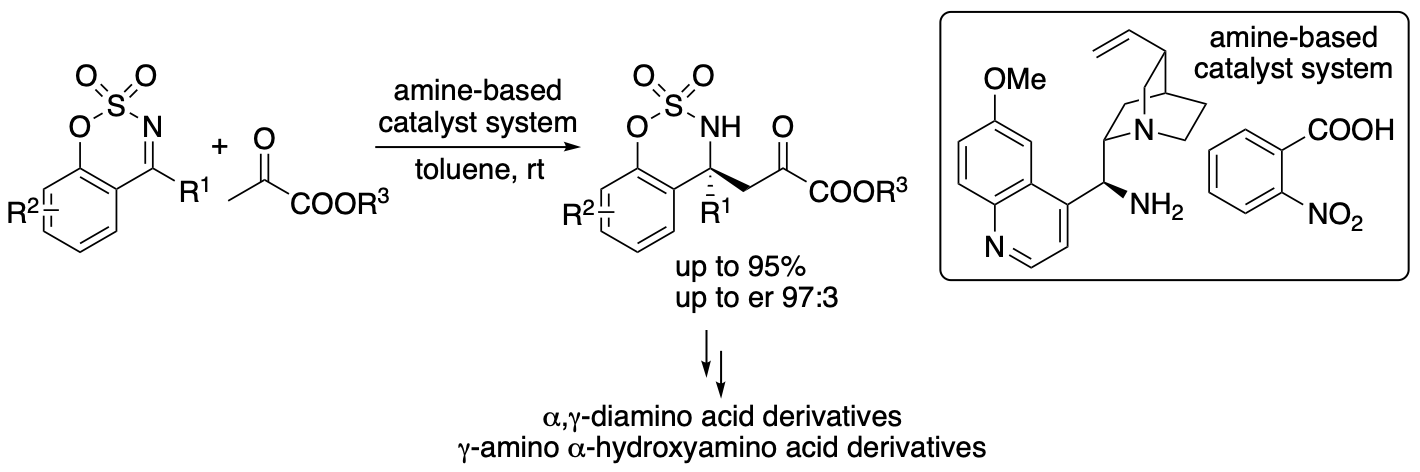
Scheme 1
To expand the strategies that enable the reactions of pyruvates as nucleophiles, we have been developing catalytic enantioselective Mannich reactions of substituted pyruvates (such as 2-oxobutanoates etc.) with cyclic and acyclic imines.
2.1.2. γ-Position selective reactions of β-ketoesters
In most aldol and Mannich reactions of β-ketoesters in which the β-ketoesters are used as nucleophiles, the bond formation occurs at the α-position of the β-ketoesters. Previously, we developed γ-position selective aldol reactions of β-ketoesters with aryl trifluoromethyl ketones catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (Scheme 2) (Zhang and Tanaka, Adv. Synth. Catal. 2015, 357, 3458). We also developed enantioselective γ-position selective aldol reactions of β-ketoesters (Zhang, Chen, Cai, Yin, Zhong, Man, Zhang, Bethi, and Tanaka, Org. Lett. 2020, 22, 6).
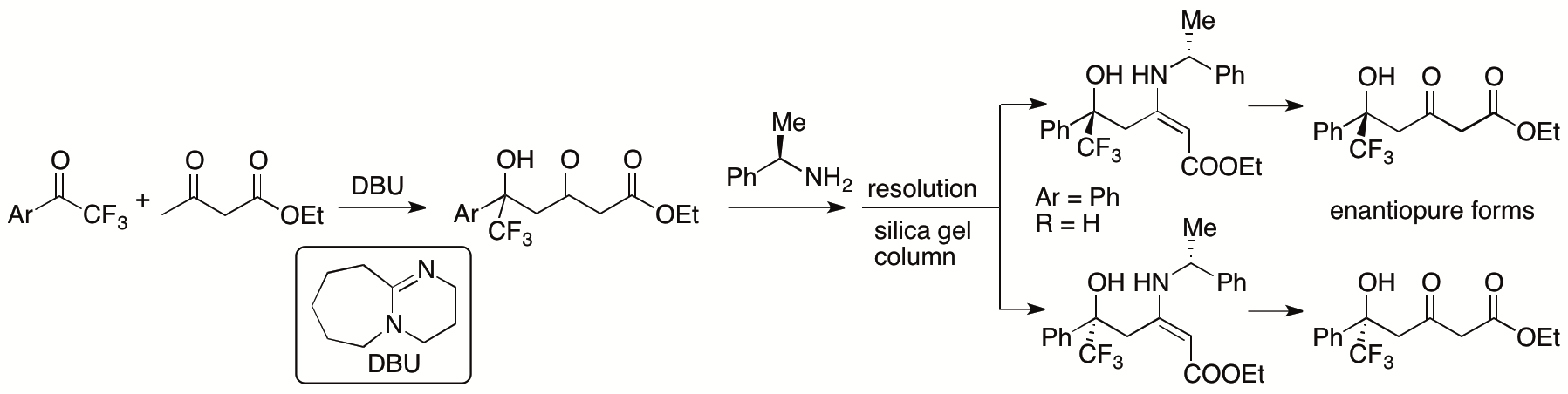
Scheme 2
With further investigations, we have developed enantioselective γ-position selective Mannich reactions of β-ketoesters (Scheme 3) (Bethi and Tanaka, Org. Lett. 2022, 24, 6711). In the developed reaction system, the bond formation at the α-position of the β-ketoester was reversible, and the γ-position-reacted product δ-amino β-ketoester derivative was kinetically formed and was stable. The dynamic kinetic process was key for the direct access to the γ-position-reacted products from β-ketocarbonyls under catalytic conditions.
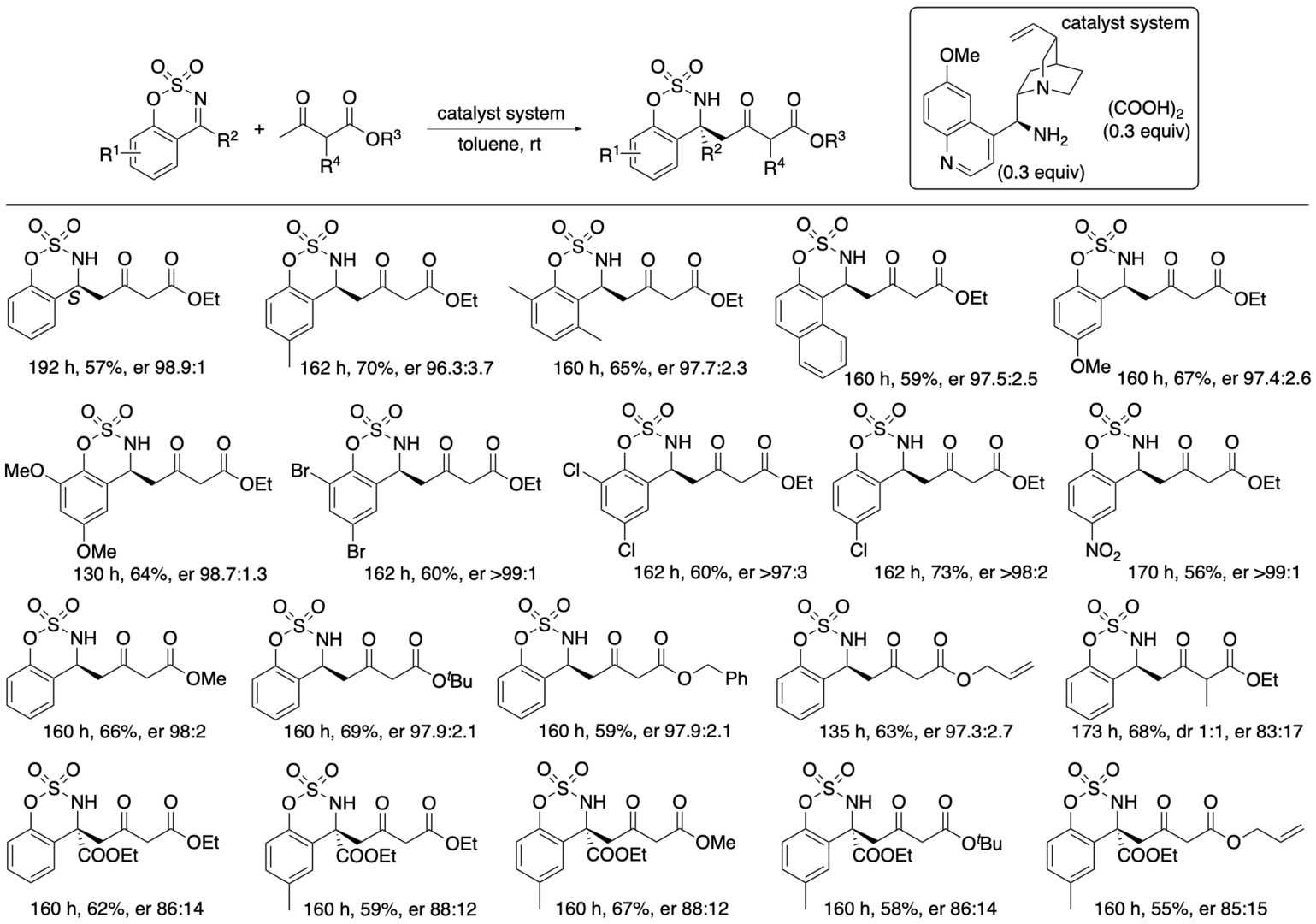
Scheme 3
We plan to expand the strategies of the γ-position selective reactions of β-ketoesters to enable to concisely perform other unusual regioselective reactions. These reactions will be useful for the synthesis of functionalized molecules under mild conditions in short routes.
2.1.3. Construction of highly functionalized decalin derivatives: aldol-aldol annulation reactions
We have recently developed catalytic enantioselective formal (4+2) cycloaddition reactions of dihydropyran derivatives with cyclohexane-1,3-diones that afford functionalized decalins through aldol-aldol annulation (Scheme 4) (Chouthaiwale, Aher, and Tanaka, Angew. Chem. Int. Ed. 2018, 57, 13298). The decalin ring system is found in diterpenes, diterpenoids, steroids, and other bioactive molecules, and thus the development of methods for the synthesis of functionalized decalin derivatives is of interest in drug discovery and related research.
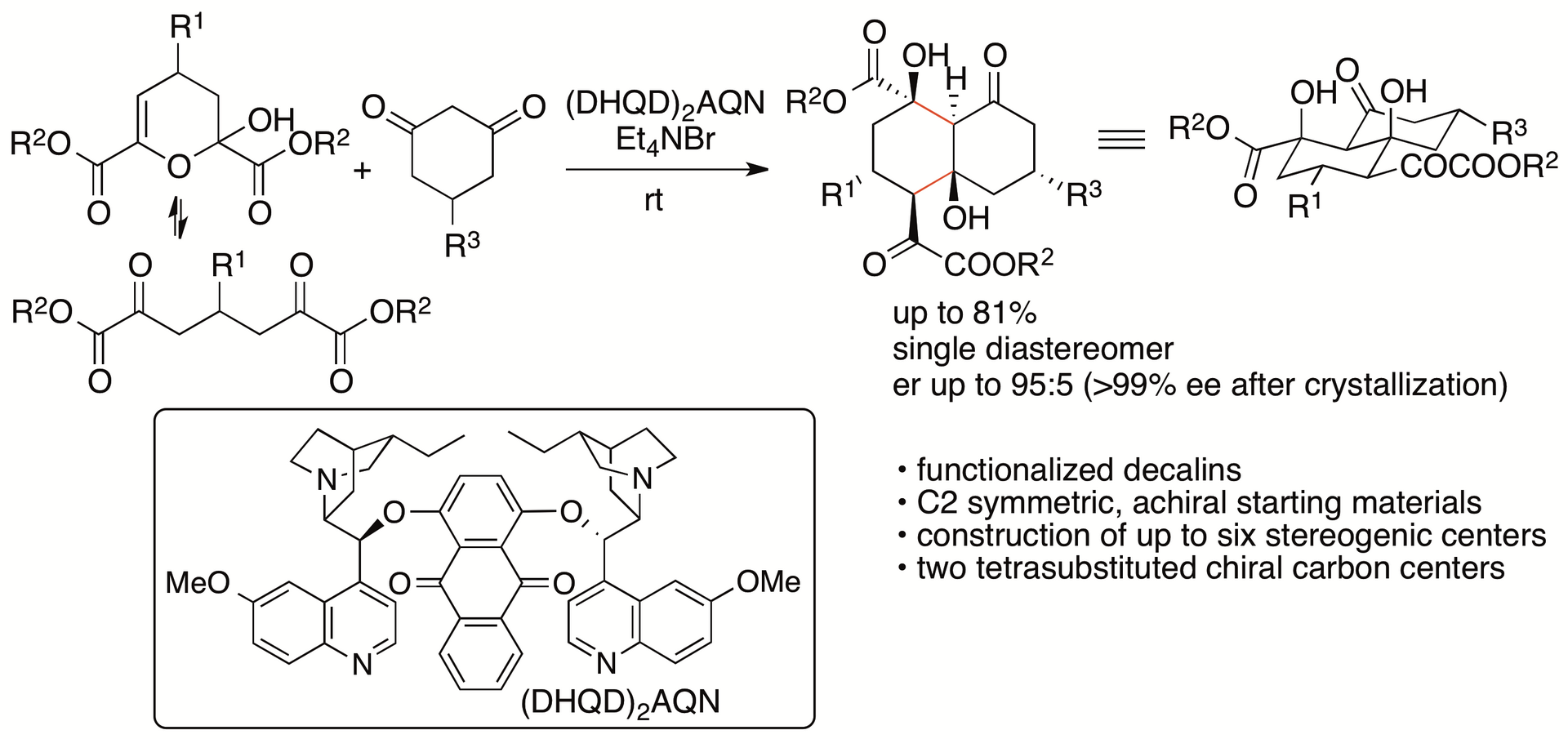
Scheme 4
Key factors of the success of the reactions include the use of the dihydropyran derivatives as the starting materials, which are obtained from pyruvates and aldehydes (Chouthaiwale and Tanaka, Chem. Commun. 2014, 50, 14881; Chouthaiwale, Lapointe, and Tanaka, Heterocycles 2017, 95, 587). The starting material dihydropyran derivatives retain the α-ketoester group of pyruvates and are C2 symmetric molecules, allowing direct enantioselective transformations to provide the products in high yields (in contrast to that kinetic resolutions result in yielding the products in no higher than 50%).
To understand the mechanisms of the reactions, including the mechanisms of the catalysis and the stereocontrol provided by the catalyst, we investigated the reactions experimentally and theoretically (Aher, Ishikawa, Yamanaka, and Tanaka, J. Org. Chem. 2022, 87, 8151). First, we have investigated the structural moieties of the catalyst necessary for the catalysis to afford the decalin products with high enantioselectivities (Scheme 5). The use of monomer-type catalyst DHQD-PHN for the reaction resulted in the formation of the decalin derivative product with the same level of the enantioselectivity as dimer-type catalyst (DHQD)2AQN that was used at the initial development shown in Scheme 4. The results indicated that moieties of DHQD-PHN, the tertiary amine, the methoxy group of the quinoline moiety, and the 9-phenanthryl group at the ether moiety, had especially important roles in the catalysis and the stereocontrol.
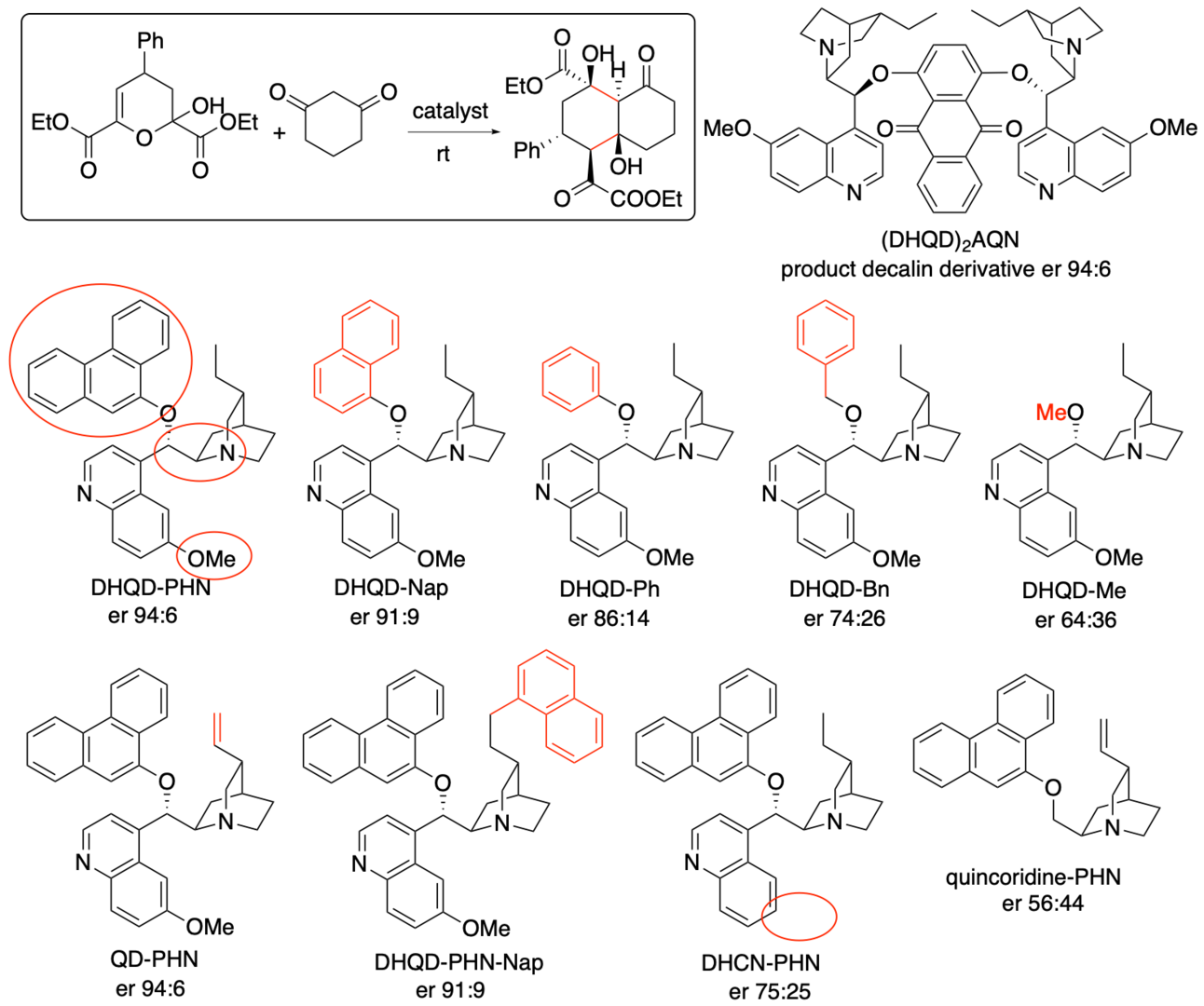
Scheme 5
We also investigated substrate substituent effects in the reaction catalyzed DHQD-PHN. In the reactions of the substrate bearing ρ-substituents on the phenyl group, the decalin products of the reactions of the substrates bearing electron-donating substituents were obtained with higher enantioselectivities (er 95:5~93:7 for methyl, methoxy, and phenyl substituents) than the products of the reactions of the substrates bearing electron-withdrawing substituents (er 90:10 and 89:11 for cyano and nitro substituents). A moderate correlation between the Hammett σp substituent constants and the enantioselectivity was observed. The results suggested that the differences in the enantioselectivities originate from the differences in the interactions between the substrates (or intermediates derived from the substrates) and the catalyst depending on the substituent during the catalysis.
We further investigated the intermediate of the reaction and the reaction using the intermediate (Scheme 6). The isolated intermediate was racemic whereas the product decalin derivative was obtained with high enantioselectivity. When racemic intermediate was treated with catalyst DHQD-PHN in the presence of 5-methylcyclohexane-1,3-dione, the usual product and the cross product were obtained with high enantioselectivities.
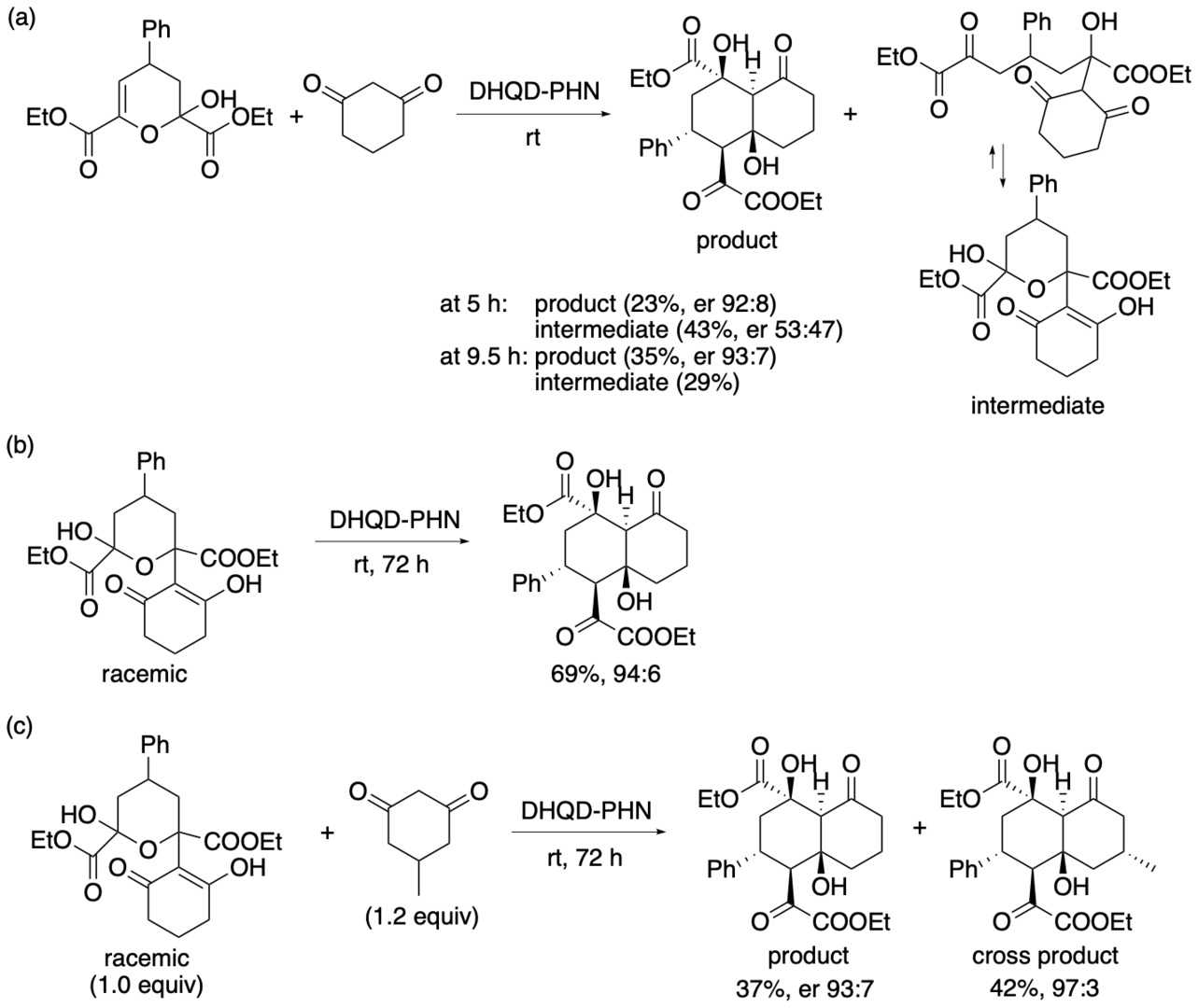
Scheme 6
These results indicate that intermediate was reversibly formed during the reaction in the presence of DHQD-PHN and that only one enantiomer of one diastereomer of the intermediate was selectively converted to the decalin derivative product. Thus, the formation of the product occurs through a dynamic kinetic desymmetrization. The results also suggest that, in the reaction in the presence of DHQD-PHN, the formation of the decalin derivative product from the intermediate is the rate-limiting step, and the stereochemistry of product is kinetically determined at this step (Scheme 7). In the DHQD-PHN-catalyzed reactions, both starting materials were desymmetrized to afford the decalin derivative products as single diastereomers with high enantioselectivities.
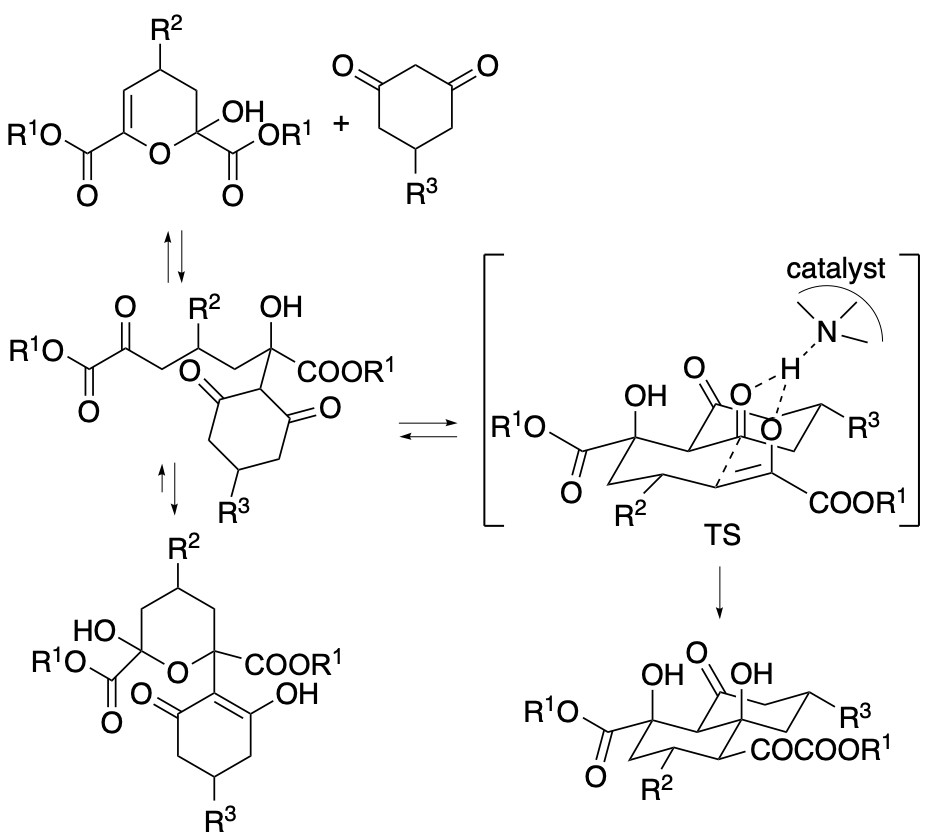
Scheme 7
Based on the experimentally obtained results, theoretical studies on the stereocontrol in the DHQD-PHN-catalyzed reaction to form the decalin derivative product (R1 = Me, R2 = Ph, and R3 = H in the structure shown in Scheme 5) were performed in collaboration with Prof. Masahiro Yamanaka, Rikkyo University. In the most stable transition state obtained in the theoretical studies, a hydrogen bond between the hydroxy group of the intermediate and the methoxy group on the quinoline moiety of DHQD-PHN and π-π stacking interactions between the phenyl group of the intermediate and the 9-phenanthryl group of the catalyst were observed in addition to the interaction between the tertiary amine moiety and the intermediate. We elucidated that the tertiary amine moiety, the methoxy group of the quinoline moiety, and the 9-phenanthryl group of DHQD-PHN are the key moieties for the catalysis and the stereocontrol.
2.1.4. Construction of spirooxindole polycyclic derivatives
Spirooxindole derivatives are found in bioactive natural products. Functionalized molecules with spirooxindole cores including those with polycyclic systems should be useful in drug discovery efforts. We previously reported formal (4+1) cycloaddition and enantioselective Michael-Henry cascade reactions that provide spirooxindole polycycles via spiro[4,5]decane derivatives (Scheme 8) (Huang, Sohail, Taniguchi, Monde, and Tanaka, Angew. Chem. Int. Ed. 2017, 56, 5853). The reactions provided spirooxindole all-carbon polycyclic derivatives with seven stereogenic centers, including two all-carbon chiral quaternary centers and one tetrasubstituted chiral carbon center.
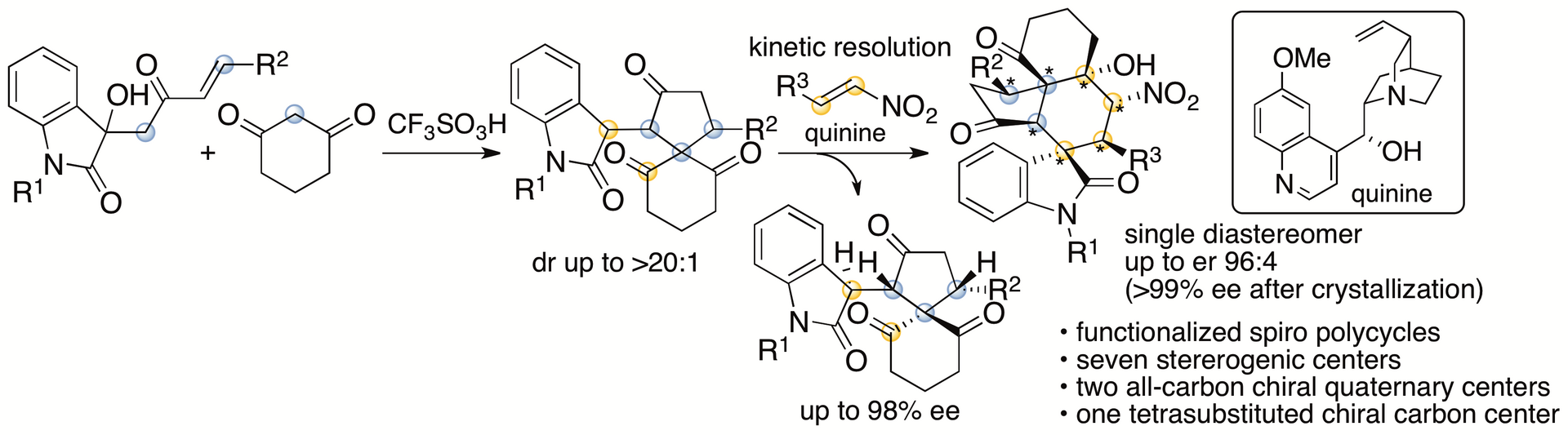
Scheme 8
Whereas kinetic resolution reactions afford the products up to 50% yield, dynamic kinetic transformations can lead to the formation of the products up to 100%. We developed dynamic kinetic transformation-based diastereo- and enantioconvergent Michael-Henry reactions that afford spirooxindoles bearing furan-fused rings (Scheme 9) (Sohail and Tanaka, Angew. Chem. Int. Ed. 2021, 60, 21256). Dihydrobenzofuranone derivatives were synthesized in racemic diastereomer mixtures, and these were transformed to the spirooxindoles bearing furan-fused polycycles with high diastereo- and enantioselectivities through the dynamic kinetic process (Scheme 9). Regardless the stereochemistry of the dihydrobenzofuranone derivative, all four isomers originated by central and axial chiralities were transformed to the single diastereomer product with high enantioselectivities.
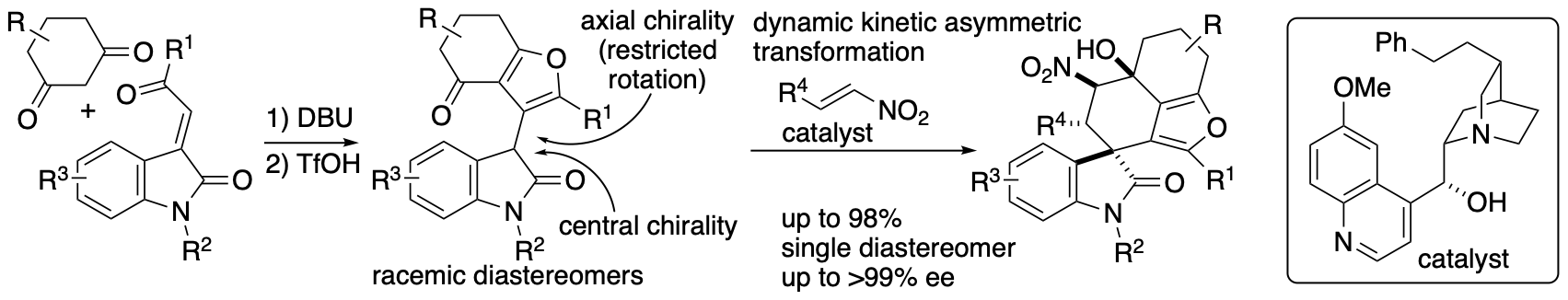
Scheme 9
Usually, these complex, polycyclic molecules are synthesized by multi step routes. Our strategies allowed the access to the complex functionalized molecules including those as highly enantiomerically enriched forms in short routes. For the reactions shown in Schemes 8 and 9, the starting materials were similar each other whereas the products were different (i.e., spiro[4,5]decanes versus dihydrobenzofuranones). We are investigation the mechanisms of the reactions and the key factors leading to the formation of these different products.
We are also investigating to provide other spiro polycyclic products and to expand the capabilities for the synthesis of functionalized molecules with spiro ring and polycyclic ring systems.
2.1.5. Design and synthesis of catalysts
We have been developing new catalysts to accelerate enantioselective chemical transformations. For example, using the Mannich reactions that we recently developed (Scheme 10) (Yuvraj and Tanaka, Org. Lett. 2020, 22, 4542), we are designing and synthesizing amine-derived catalysts that have contiguous chiral centers.
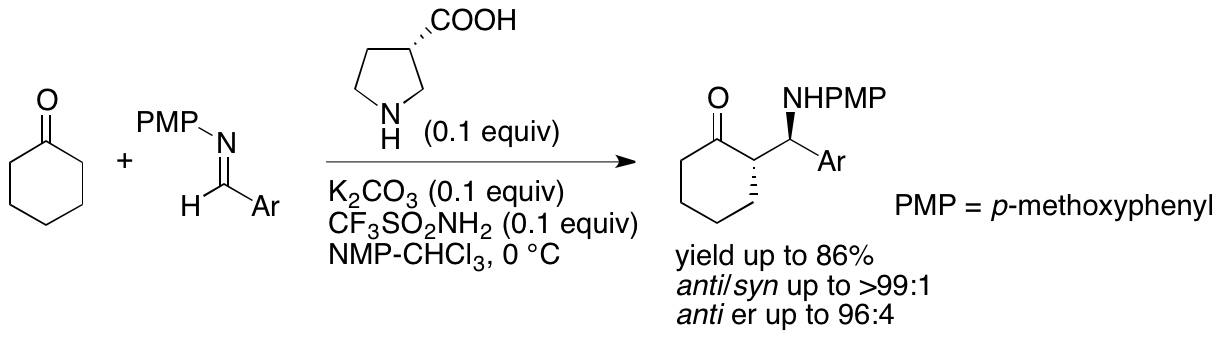
Scheme 10
2.1.6. Control of chemical reactions using 1,3-cyclohexanedione derivatives as buffering molecules in non-aqueous solutions
Control of chemical reactions is necessary to obtain desired chemical transformation products and to prevent decompositions and isomerizations of molecules of interest. To control chemical reactions in aqueous solutions or to maintain conditions suitable for enzyme-catalyzed reactions and for storage of biological samples such as enzymes and antibodies, the use of buffers is a common practice. We have introduced the "buffering" concept into the events that occur in non-aqueous solutions and have demonstrated that 1,3-cyclohexanedione derivatives have buffering functions in non-aqueous solutions (Scheme 11) (Sohail and Tanaka, Chem. Eur. J. 2020, 26, 222). We have demonstrated that 1,3-cyclohexanedione derivatives inhibit both acid-catalyzed and base-catalyzed isomerizations and decompositions in organic solvents. To suppress decompositions and isomerizations of the molecules by using the buffering molecules that we have disclosed, there is no need to consider whether the decomposition and/or isomerization is caused by a base or an acid or which type of base or acid is causing the decomposition, isomerization, and/or racemization. Additionally, simply adding the buffering molecule to reaction mixtures can completely alter the pathways of the reactions.
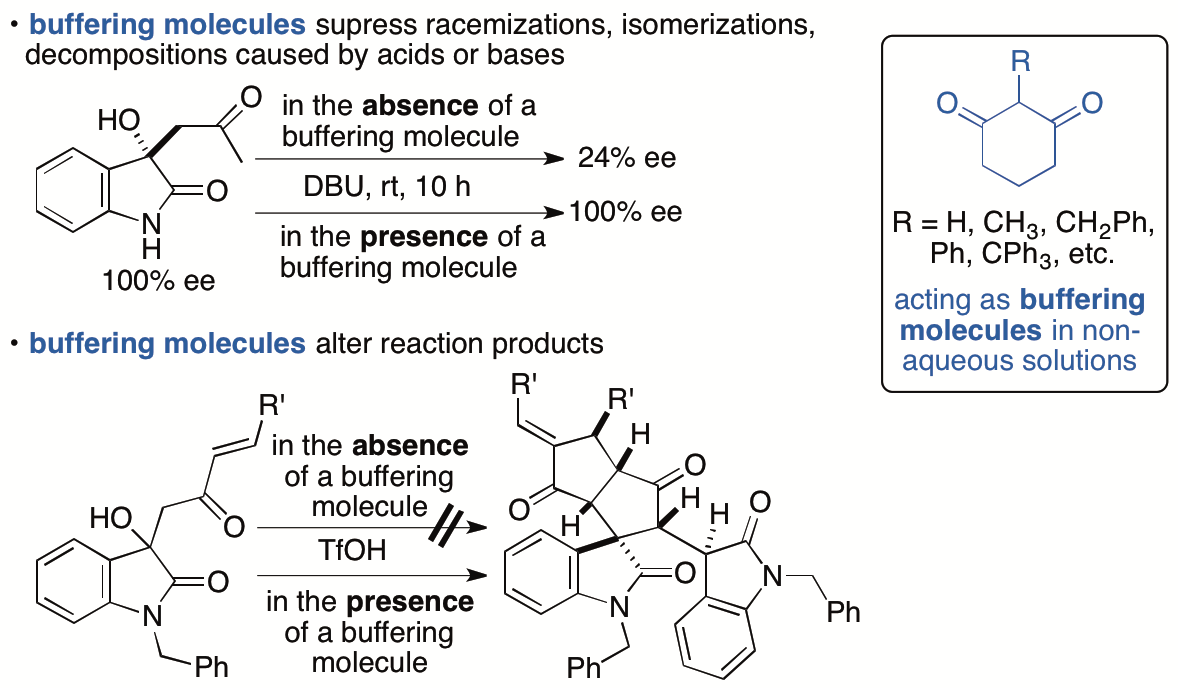
Scheme 11
We are expanding the concept for tuning the reactivities in organic solvents. We are also developing reaction systems and catalyst systems that use the buffering functions of 1,3-cyclohexanedione derivatives.
2.2 Development of bioconjugation systems
Protein labeling methods are required for the synthesis of antibody-drug conjugates and other protein conjugates; these molecules are important as therapeutics and as detection devices for molecules of interest. Conjugation reactions are also needed to create multifunctional molecules. We are developing efficient protein labeling systems and molecules with desired reactivities that can be used for protein labeling reactions at targeted sites.
2.3 Search of biofunctional molecules
As described above, we have synthesized various functionalized molecules. In collaboration with researchers whose expertise is in biology and screening for biofunctional molecules, we have been searching new biofunctional molecules and drug leads.
3. Publications
3.1 Journals
- Aher, R. D.; Ishikawa, A.; Yamanaka, M.; Tanaka, F. Catalytic Enantioselective construction of decalin derivatives by dynamic kinetic desymmetrization of C2-symmetric derivatives through aldol-aldol annulation. The Journal of Organic Chemistry 2022, 87, 8151-8157, doi: 10.1021/acs.joc.2c00889.
URL: https://pubs.acs.org/doi/full/10.1021/acs.joc.2c00889
or
URL: https://pubs.acs.org/articlesonrequest/AOR-MD3RUK7QPZFDIYFEDJMY
- Bethi, V.; Tanaka, F. Organocatalytic enantioselective γ-position-selective Mannich reactions of β-ketocarbonyl derivatives. Organic Letters 2022, 24, 6711-6715, doi: 10.1021/acs.orglett.2c02433.
URL: https://pubs.acs.org/doi/full/10.1021/acs.orglett.2c02433
- Tanaka, F. Amines as catalysts: dynamic features and kinetic control of catalytic asymmetric chemical transformations to form C-C bonds and complex molecules. The Chemical Record, doi 10.1002/tcr.202200207.
URL: https://onlinelibrary.wiley.com/doi/full/10.1002/tcr.202200207
- Sohail, M.; Tanaka, F. Synthesis of functionalized spirooxindole polycycles: Use of cyclic 1,3-diones as reactants or as condition-tuning molecules. Synlett in press, doi 10.1055/a-2061-0855.
URL: https://www.thieme-connect.com/products/ejournals/html/10.1055/a-2061-0855
3.2 Oral and Poster Presentations
- Garg, Y.; Tanaka, F. Enantioselective Mannich reactions of ketones catalyzed by 3-pyrrolidinecarboxylic acid in the presence of metal salts, in Indo-African International Conference on Emerging Materials Science and Technologies (IAFICEMST) 2022, online, 2022.08.03-2022.08.05 (invited talk (Garg, Y.), No. 63)
- Mondal, S.; Tanaka, F. Organocatalytic direct enantioselective Mannich reactions of pyruvates as nucleophiles, in the ACS Spring 2023, Indianapolis, USA, 2023.03.26-2023.03.30. (oral presentation No. ORGN 3812977)
- Sohail, M.; Tanaka, F. Control of Chemical Reactions in Non-aqueous Solutions Using Buffering Molecules, in The 143rd Annual Meeting of the Pharmaceutical Society of Japan, Hiroshima, Japan (2023), 2023.03.25-2023.03.28. (poster No. 27P1-pm1-018)
4. Intellectual Property Rights
- Tanaka, F.; Johnson, S., Oligosaccharide C-glycoside derivatives, patent granted, US11401293 B2, 2022.08.02.



