FY2016 Annual Report
Chemistry and Chemical Bioengineering Unit
Professor Dr. Fujie Tanaka, Associate Professor
Abstract
The ability to design and synthesize organic molecules constitutes the foundation that underlies basic research as well as applied science. The ability is also essential for the development of pharmaceuticals and biofunctional molecules. This unit develops new, efficient, concise, and safe chemical transformation methods and strategies for constructing small molecules bearing functional groups and/or chiral centers that are relevant to the creation of biofunctional molecules. We design and create small organic molecule catalyst systems that enable designed chemical transformations, and we develop reaction strategies that use small organic molecules as enzyme-like catalysts. With the use of organic molecules as catalysts, we minimize the need for protection and deprotection steps that are usually required for the synthesis of functionalized molecules. When a reaction method does not affect functional groups that are not at the reaction site, the reaction method can be used for the synthesis of a series of molecules bearing various functional groups. This means that a series of molecules of interest may be synthesized using the same method in a short route, providing advantages for the synthesis of biofunctional candidate molecules. We also investigate the chemical bases of the reactions to understand the mechanisms of the catalysis and molecular interactions provided by organic molecules. By taking advantage of the use of features of our developing molecules, we also develop strategies and methods for conjugation of proteins and peptides with other molecules. The molecules that we have synthesized are screened in various functional assays in collaboration with other research groups. The research undertaken by this unit advances the chemistry of catalysis and of molecular synthesis. The studies by this unit accelerate the creation of molecules used in biomedical research and contribute to the development of new therapeutics, therapeutic strategies, and diagnostic methods.
1. Staff
- Dr. Ravindra D. Aher
- Dr. Avik Kumar Bagdi
- Dr. Santosh Chavan
- Dr. Pandurang V. Chouthaiwale
- Dr. Jithender Enukonda
- Swetha Enukonda
- Dr. Sherida Johnson
- Dr. Yarkali Krishna
- Dr. Lingaiah Maram
- Dr. Prodip M. Roy
- Dr. Muhammad Sohail
- Dr. Feng Yin
- Dongxin Zhang, Graduate Student
- Ainash Garifullina, Graduate Student (Rotation)
- Soumen Jana, Graduate Student (Rotation)
- Dong Cao, Graduate Student (Early start)
- Hien Thu Chu Thi, Research Intern
- Maira Pasha, Research Intern
- Shiho Chinen, Research Unit Administrator
2. Activities and Findings
2.1 Development of new chemical transformation methods and synthesis of functionalized molecules
We have been developing small organic molecule catalysts (organocatalysts) and organocatalytic molecular transformation methods useful for the synthesis of functionalized molecules under mild conditions in short routes. We also investigate the chemical basis of the catalysis and the chemical transformations to understand the mechanisms of the catalysis and molecular interactions provided by organic molecules to further the creation of useful molecules.
Traditional synthetic methods often require high or very low temperatures and/or absolute conditions. In addition, functional groups on substrate molecules must be protected prior to reactions. That is, depending on functional groups present in target molecules to be synthesized, synthetic routes, including protection and deprotection steps, have to be designed for each molecule. To concisely synthesize functionalized molecules, chemical transformation methods that are not affected by functional groups presenting in starting materials are needed. It is a great advantage when the same reaction method can be used for the synthesis of a series of molecules bearing various functional groups without the need of product-specific protection and deprotection steps. In addition, it is desired that such reactions can be performed under safe, mild, and environmentally benign conditions. We address these points in our research as we design and develop catalysts and chemical transformation methods. By using organic molecules as catalysts, we concisely synthesize novel functionalized molecules including those that are often difficult to synthesize by traditional synthetic strategies. Our studies provide molecules that are screened for bioactive candidates and contribute to the creation of new functional molecules. Our investigations into the chemical basis of the developed catalysts and chemical transformation methods further the understanding of the chemistry of organic molecules and their reactions.
2.1.1. Asymmetric construction of spirooxindole polycycles
One of the recent achievements of this unit is the development of formal (4+1) cycloaddition and enantioselective Michael-Henry cascade reactions that provide spirooxindole polycycles (Scheme 1) (Huang, Sohail, Taniguchi, Monde, and Tanaka, Angew. Chem. Int. Ed. 2017, 56, 5853). Spiro[4,5]decanes and polycyclic compounds bearing spiro[4,5]decane systems are found in bioactive natural products. Functionalized molecules with these cyclic systems should be useful in drug discovery efforts. We have developed formal (4+1) cycloaddition reactions that afford spiro[4,5]decane derivatives bearing oxindole moieties. We have also developed diastereo- and enantioselective Michael-Henry-cascade reactions of the (4+1) cycloaddition products that afford spirooxindole polycyclic derivatives bearing the spiro[4,5]decane system with seven stereogenic centers, including two all-carbon chiral quaternary centers and one tetrasubstituted chiral carbon center. Our strategies allow access to these complex functionalized molecules in highly enantiomerically enriched forms in two steps.
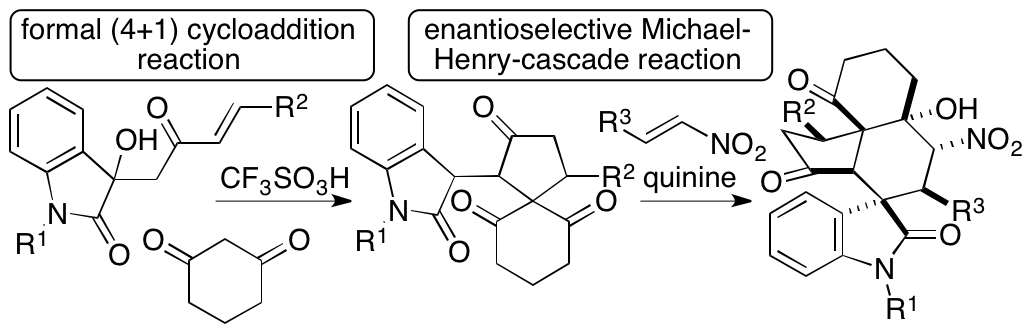
Scheme 1
We are currently investigating the mechanisms of the formal (4+1) cycloaddition reaction that provides the spiro[4,5]decane ring system. We are also investigating the mechanisms of the reactions of the spiro[4,5]decane derivatives and expanding the capabilities for the synthesis of functionalized molecules with polycyclic ring systems.
2.1.2. Asymmetric oxa-hetero-Diels-Alder reactions: Synthesis of spirooxindole tetrahydropyran derivatives
We recently reported catalytic asymmetric hetero-Diels-Alder reactions of enones with isatins (2,3-dioxyindoles or 2,3-dioxyindolins) that provide functionalized spirooxindole tetrahydropyranones with high diastereo- and enantioselectivities (Scheme 2) (Cui and Tanaka, Chem. Eur. J. 2013, 19, 6213). Novel amine-based catalyst systems composed of three-types of molecules (amine, acid, and thiourea) were developed to catalyze the reactions. The design and synthesis of single-molecule catalysts that provide all the required interactions for the catalysis and stereocontrol for designed reactions especially for new reactions are often difficult. We have demonstrated that the use of multicomponent catalyst systems provides a way to bypass the limitations in the access to efficient single component catalysts. We also elucidated the mechanism of the reactions and key factors for the high diastero- and enantioselectivities achieved by the three-component catalyst system (Cui, Chouthaiwale, Yin, and Tanaka, Asian J. Org. Chem. 2016, 5, 153).
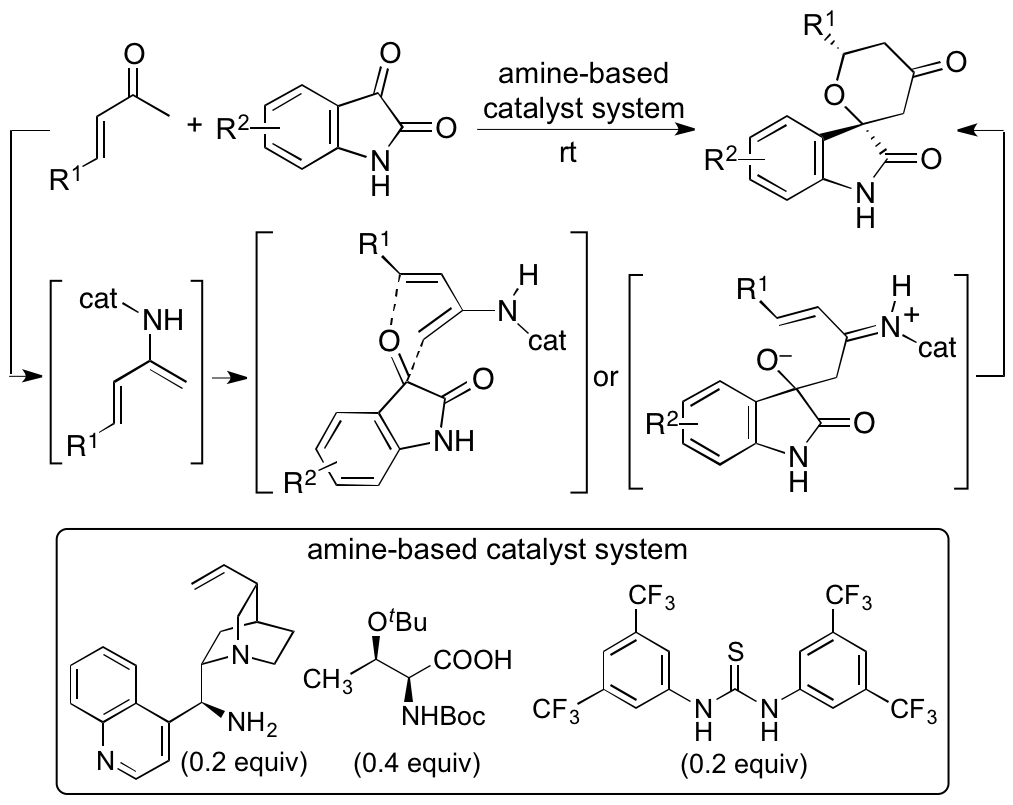
Scheme 2
With the developed reaction method and with derivatization of the hetero-Diels-Alder reaction products, various spirooxindole tetrahydropyranone derivatives were synthesized (Cui, Chouthaiwale, Yin, and Tanaka, Org. Biomol. Chem. 2016, 14, 259). In collaboration with Professor Tomoda, Kitasato University, SOAT2 inhibitors were identified from the synthesized spirooxindole tetrahydropyranone derivatives (Kobayashi, Ohshiro, Tomoda, Yin, Cui, Chouthaiwale, and Tanaka, Bioorg. Med. Chem. Lett. 2016, 26, 5899). We are investigating to expand the ability to provide biofunctional molecules through the use of the spirooxindole tetrahydropyranone structure obtained from the hetero-Diels-Alder reactions and derivatizations of the products. We are also expanding the hetero-Diels-Alder reactions of enones beyond the use of the oxindole dienophiles to the use of various ketones and aldehydes as dienophiles.
2.1.3. Cascade reactions of pyruvates with aldehydes to synthesize various functionalized molecules
Pyruvates can act as nucleophiles and electrophiles and thus are expected to be useful synthons. However, the dual reactivities of pyruvates are difficult to control. We have recently developed concise cascade reactions of pyruvates to provide various functionalized dihydropyrans in one pot under mild conditions (Scheme 3) (Chouthaiwale and Tanaka, Chem. Commun. 2014, 50, 14881). Further, we have demonstrated that the product dihydropyrans are readily transformed to various molecules including amino group-substituted and fluoro group-substituted dihydropyrans, cyclohexane-derived amino acids, dihydrodiazepines, and pyridines. To further simplify the synthesis, we developed a one-pot method; 4-substituted-pyridine-2,6-dicarboxylic acid derivatives were synthesized in one pot from pyruvates and aldehydes (Chouthaiwale, Lapointe, and Tanaka, Heterocycles 2017, 95, 587).
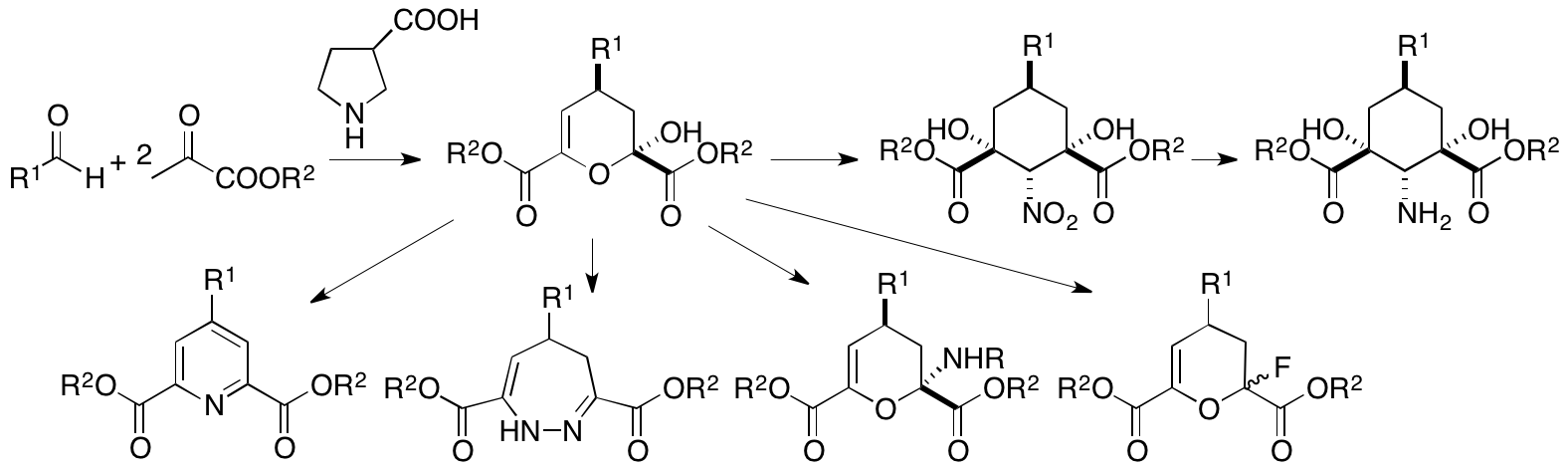
Scheme 3
We are further developing transformation methods of the dihydropyrans obtained from pyruvates and aldehydes, including enantioselective methods in order to concisely provide enantiomerically enriched functionalized molecules.
2.1.4. Direct C-C bond-forming reactions of unprotected carbohydrates
Carbohydrate backbone-elongation reactions at anomeric carbons are important for the synthesis of higher carbon-backbone carbohydrates and of C-glycosides. Generally, reactions on carbohydrates require protection of hydroxy groups that are later deprotected. If unprotected carbohydrates could be used as substrates, the methods would be atom- and step-economical. There are only limited numbers of non-enzymatic direct C-C bond forming reactions at the anomeric carbon of unprotected aldoses that are mainly present as cyclic hemiacetals. We have recently developed direct C-glycoside formation reactions of unprotected 2-N-acetyl-aldohexoses via aldol condensation-oxa-Michael reactions catalyzed by amine-based catalysts (Johnson and Tanaka, Org. Biomol. Chem. 2016, 14, 259). Currently we are developing C-C bond formation reaction methods of unprotected di- and trisaccharides. We are continuing to develop carbohydrate backbone-elongation reactions of unprotected carbohydrates, including the reactions of unprotected oligosaccharides.
2.1.5. DBU-catalyzed aldol and other reactions
We have recently developed DBU-catalyzed aldol reactions (Zhang, Johnson, Cui, and Tanaka, Asian J. Org. Chem. 2014, 3, 391; Zhang and Tanaka, Adv. Synth. Catal. 2015, 357, 3458). In these DBU-catalyzed aldol reactions, the C-C bonds formed in perfect regioselectivities at the methyl groups of alkyl methyl ketones, at the g-positions of b-keto esters, and at the methyl groups of the b-methyl-substituted cyclic enones (Scheme 4). For the aldol products from the b-keto esters, enantiomerically pure forms were obtained by the resolution of the enamines of the aldol products with a homochiral amine. As a next stage, we are designing and synthesizing DBU-inspired chiral versions of catalysts to perform enantioselective aldol reactions and related reactions.
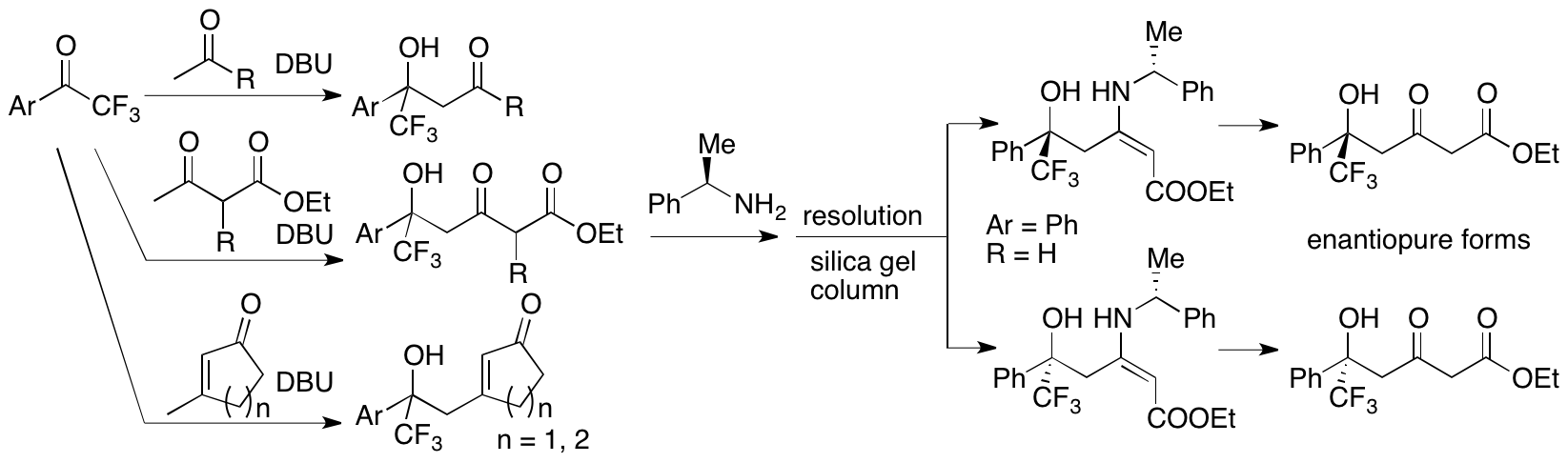
Scheme 4
2.1.6. Mechanisms of the catalysis and regioselectivities of aldol reactions
As described above, we have shown that the DBU catalysis provides notable regioselectivities in aldol reactions. In the catalyzed reactions of ketones, the relationship between the formation of enolates or enamines and the formation of products, including regioselective formation of products, is not well understood. When a ketone has two enolizable a-positions, the carbanion may form at both the a-positions or at either of the a-positions. Regioselectivities of catalyzed aldol and related reactions may be the results of regioselective formation of an enolate/enamine or because a single type of enolates/enamines among those formed results in product formation. To provide insight into aldol reaction catalysis, the relative frequencies of carbanion formation at each a-position of ketones under catalysis by DBU, proline, and related catalysts were determined through the deuteration of the ketones in the presence of these catalysts (Scheme 5) (Zhang and Tanaka, Org. Lett. 2017, 19, 3803). For the reaction of 1,1-dimethoxypropan-2-one, the deuterated site matched the C-C bond formation site in the aldol reactions, and faster reaction by the DBU catalysis than that by proline catalysis was in accord with the faster deuteration rate by DBU than that by proline catalysis. For other ketones tested in this study, such as 2-pentanone, methoxyacetone, hydroxyacetone, and ethyl 3-oxobutanoate, however, the positions favored for deuteration did not directly correlate with the C-C bond formation sites in aldol and related reactions. Formation of carbanions (or enolates/enamines) is necessary to be the reaction site, and for fast reactions, rapid or frequent enolate/enamine formation is required. But, the results indicate that frequent carbanion formation site is not necessary to be the C-C bond formation site. Through this study, mechanisms of regioselectivities of the reactions and mechanisms of catalysis were clarified to some degree.
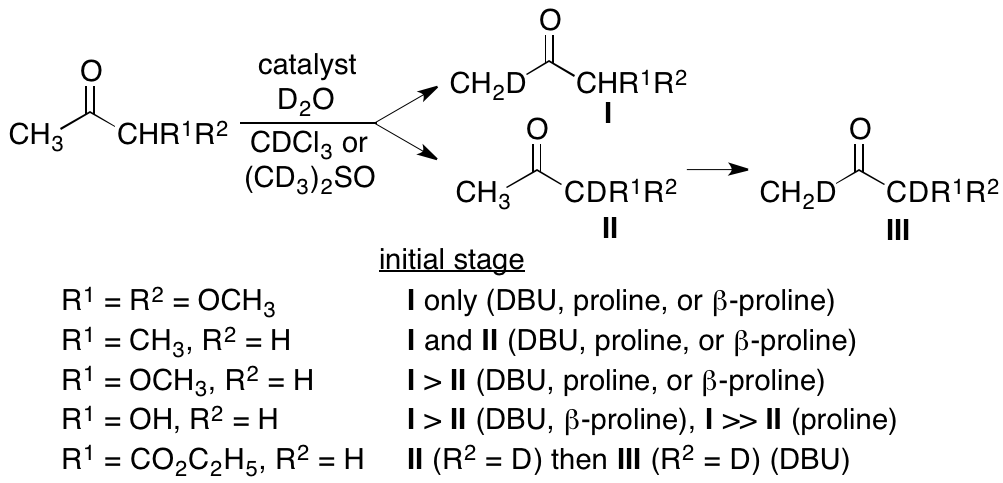
Scheme 5
2.1.7. Asymmetric Michael reactions: Synthesis of pyrrolidine-3-carboxylic acid derivatives
Pyrrolidine-3-carboxylic acid (b-proline) derivatives and b2-amino acids are important molecules as bioactives, catalysts for chemical transformations, and their building blocks. To concisely synthesize these compounds, we have developed organocatalytic enantioselective Michael reactions of 4-alkyl-substituted-4-oxo-2-enoates with nitroalkanes (Scheme 6) (Yin, Garifullina, and Tanaka, Org. Biomol. Chem. 2017, 15, 6089). Using the developed method, highly enantiomerically enriched 5-methylpyrrolidine-3-carboxylic acid was synthesized in two steps from easily accessible starting materials.
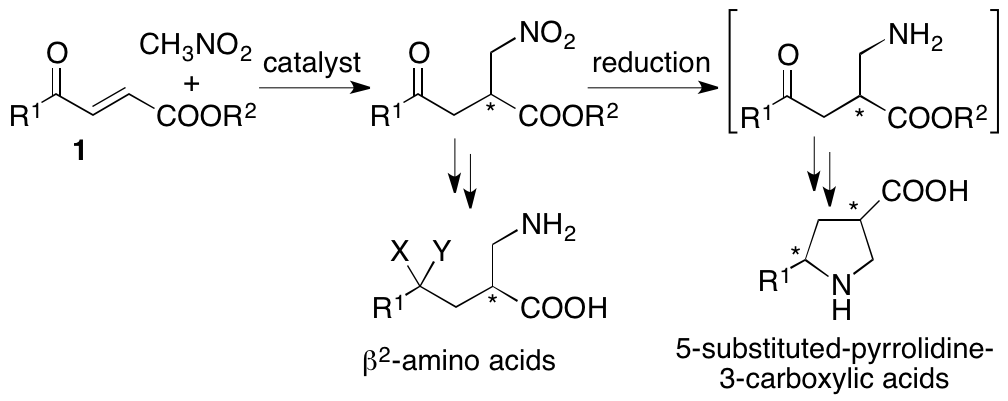
Scheme 6
2.2. Fluorescence-based reaction monitoring systems
This unit has also been developing concise fluorescence-based assay methods to monitor bond-forming and bond-breaking reaction progress on a small scale to facilitate the development of catalysts and catalyzed chemical transformation methods. Use of fluorogenic substrates provides a straightforward method of reaction monitoring because reaction progress is directly observed as an increase in fluorescence.
For example, we have developed fluorogenic aldehydes based on a diarylacetylene core structure and fluorescence-based assay systems for aldol reactions using the fluorogenic aldehydes (Scheme 7) (Katsuyama, Chouthaiwale, Akama, Cui & Tanaka, Tetrahedron Lett. 2014, 55, 74). We are continuing to develop fluorogenic substrates and fluorescence-based assay systems with improved features.
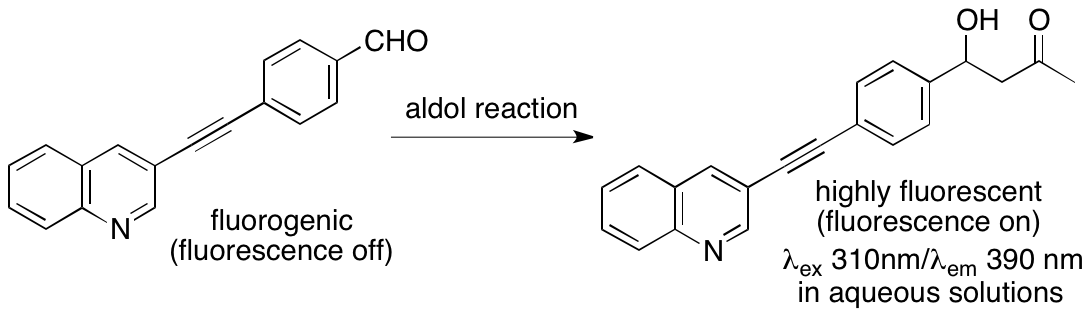
Scheme 7
2.3. Development of bioconjugation systems
Protein labeling methods are required for the synthesis of antibody-drug conjugates and other protein conjugates; these molecules are important as therapeutics and as detection devices for molecules of interest. Conjugation reactions are also needed to create multifunctional molecules. We are developing efficient protein labeling systems and molecules with desired reactivities that can be used for protein labeling reactions at targeted sites.
3. Publications
3.1. Journals
- Zhang, D.; Tanaka, F. Catalytic enantioselective oxa-hetero-Diels-Alder reactions of enones with aryl trifluoromethyl ketones. RSC Advances, 6, 61454-61457 (2016), doi: 10.1039/c6ra13859d.
- Chouthaiwale, P. V.; Lapointe, S.; Tanaka, F. Synthesis of 4-substituted-pyridine-2,6-dicarboxylic acid derivatives from pyruvates and aldehydes in one pot. Heterocycles, 95, 587-594 (2017), doi: 10.3987/COM-16-S(S)27.
- Kobayashi, K.; Ohshiro, T.; Tomoda, H.; Yin, F.; Cui, H.-L.; Chouthaiwale, P. V.; Tanaka, F. Discovery of SOAT2 Inhibitors from Synthetic Small Molecules. Bioorganic & Medicinal Chemistry Letters, 26, 5899-5901 (2016), doi: 10.1016/j.bmcl.2016.11.008.
- Huang, J.-R.; Sohail; M.; Taniguchi, T.; Monde, K.; Tanaka, F. Formal (4+1) cycloaddition and enantioselective Michael-Henry-cascade reactions to synthesize spiro[4,5]decanes and spirooxindole polycycles. Angewandte Chemie Internal Edition, 56, 5853-5857 (2016), doi: 10.1002/anie.201701049. (German Edition doi: 10.1002/ange.201701049)
- Zhang, D.; Tanaka, F. Determination of relative frequency of carbanion formation at a-positions of ketones under aldol reaction catalysis conditions. Organic Letters 19, 3803-3806 (2017), doi: 10.1021/acs.odglett.7b01676.
- Yin, F.; Garifullina, A.; Tanaka, F. Synthesis of pyrrolidine-3-carboxylic acid derivatives via asymmetric Michael addition reactions of carboxylate-substituted enones. Organic & Biomolecular Chemistry 15, 6089-6092 (2017), doi: 10.2039/c7ob01484h.
3.2. Book Chapters
- Tanaka, F. Enzymes and organocatalysts, in CSJ Current Review 22 The Chemistry of Organocatalysis, The Chemical Society of Japan, Kagakudojin, 2016. 田中富士枝,酵素と有機分子触媒,CSJ カレントレビュー 22 有機分子触媒の化学,日本化学会編,化学同人 2016年
3.2. Oral and Poster Presentations
- Zhang, D. & Tanaka, F. Catalytic enantioselective hetero-Diels-Alder reactions of enones with aryl trifluoromethyl ketones to give trifluoromethyl-substituted tetrahydropyranones, in Molecular Chirality Asia 2016, Osaka, Japan, 2016.04.20-2016.04.22.
- Maram, L.; Tanaka, F. Synthesis of iminosugar C-glycosides via a Mannich reaction-aminocyclization route, in Molecular Chirality Asia 2016, Osaka, Japan, 2016.04.20-2016.04.22.
- Zhang, D.; Tanaka, F. Deuteration studies of DBU-catalyzed aldol reactions, in The 9th Symposium on Organocatalysis, Nagoya, Japan, 2016.12.01-2016.12.02. (poster No. P15)
- Zhang, D.; Tanaka, F. Construction of aryl- and trifluoromethyl-substituted tertiary alcohols via aldol reactions catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene, in ICOS 21, 21st International Conference on Organic Synthesis, IIT Bombay, Mumbai, India, 2016.12.11-2016.12.16. (poster No. P99) (international conference)
- Maram, L.; Tanaka, F. Synthesis of iminosugar C-glycosides via Mannich-aminocyclization reactions, in The 137th Annual Meeting of the Pharmaceutical Society of Japan, Sendai, Japan (2017), 2017.03.24-2017.03.27. (poster No. 25PA-am126)
- Tanaka, F. Organocatalytic reactions for the synthesis of functionalized molecules, in The 7th Symposium on Biomolecular Science in OPU, Osaka, Japan (2017), 2017.03.28. (oral, invited talk)
- Zhang, D.; Tanaka, F. DBU-catalyzed regioselective aldol reactions to synthesize functionalized molecules, in The 45th ACS National Organic Chemistry Symposium (NOS 2017), UC Davis, US, 2017.06.25-2017.06.29. (poster No. W56) (international conference)
- Maram, L.; Tanaka, F. Stereoselective synthesis of iminosugar C- glycosides via a Mannich-aminocyclization route, in Chirality 2017; 29th International Symposium on Chirality, Tokyo, 2017.07.09-2017.07.12. (poster No. P-130) (international conference)
- Sohail, M.; Huang, J.-R.; Tanaka, F. Enantioselective synthesis of functionalized spiro[4,5]decanes and spirooxindole polycycles by formal (4+1) cycloaddition and Michael Henry-cascade reactions, in Chirality 2017; 29th International Symposium on Chirality, Tokyo, 2017.07.09-2017.07.12. (poster No. P-132) (international conference)
- Sohail, M.; Huang, J.-R.; Tanaka, F. Two steps, (4+1) cycloaddition and kinetic resolution by Michael Henry-cascade reactions, leading to highlyfunctionalized enantiomerically enriched spiro[4,5]decanes and spirooxindole polycycles, in the 254th ACS National Meeting, Washington, DC, US, 2017.08.20-2017.08.24. (oral presentation No. ORGN 488) (international conference)
4. Seminars
4.1. Seminars Hosted
- Date: February 14, 2017
- Venue: OIST campus
- Speaker: Prof. Yong-Qiang Tu, Lanzhou University and Shanghai Jiao Tong University, China
- Date: February 16, 2017
- Venue: OIST campus
- Speaker: Prof. Yong-Qiang Tu, Lanzhou University and Shanghai Jiao Tong University, China
- Date: March 17, 2017
- Venue: OIST campus
- Speaker: Prof. Santos Fustero, University of Valencia, Spain



