FY2018 Annual Report
Chemistry and Chemical Bioengineering Unit
Professor Dr. Fujie Tanaka, Associate Professor
Abstract
The ability to design and synthesize organic molecules constitutes the foundation that underlies basic research as well as applied science. The ability is also essential for the development of pharmaceuticals and biofunctional molecules. This unit develops efficient, concise, and safe chemical transformation methods and strategies for constructing small molecules bearing functional groups and/or chiral centers that are relevant to the creation of biofunctional molecules. We design and create small organic molecule catalyst systems for designed chemical transformations, and we develop reaction strategies that use small organic molecules as enzyme-like catalysts. With the use of organic molecules as catalysts, we minimize the need for protection and deprotection steps that are usually required for the synthesis of functionalized molecules. When a reaction method does not affect functional groups that are not at the reaction site, the reaction method can be used for the synthesis of a series of molecules bearing various functional groups. This means that a series of molecules of interest may be synthesized using the same method in a short route, providing advantages for the synthesis of biofunctional candidate molecules. We also investigate the chemical bases of the reactions to understand the mechanisms of the catalysis and molecular interactions provided by organic molecules. By taking advantage of the use of features of our developing molecules, we also develop strategies and methods for conjugation of proteins and peptides with other molecules. The research undertaken by this unit advances the chemistry of catalysis and of molecular synthesis. The studies by this unit accelerate the creation of molecules used in biomedical research and contribute to the development of new therapeutics, therapeutic strategies, and diagnostic methods.
1. Staff
- Dr. Ravindra D. Aher
- Dr. Venkati Bethi
- Dr. Santosh Chavan
- Dr. Yuvraj Garg
- Dr. Yarkali Krishna
- Dr. Lingaiah Maram
- Dr. Prodip M. Roy
- Dr. Muhammad Sohail
- Kola Shilpa
- Santanu Mondal, Graduate Student (Rotation)
- Maira Pasha, Special Research Student
- Shiho Chinen, Research Unit Administrator
2. Activities and Findings
2.1. Development of new chemical transformation methods and synthesis of functionalized molecules
We have been developing small organic molecule catalysts (organocatalysts) and organocatalytic molecular transformation methods useful for the synthesis of functionalized molecules under mild conditions in short routes. We also investigate the chemical bases of the catalyses and the chemical transformations to understand the mechanisms of the catalysis and molecular interactions provided by organic molecules to further the creation of useful molecules.
Traditional synthetic methods often require high or very low temperatures and/or absolute conditions. In addition, functional groups on substrate molecules must be protected prior to reactions. That is, depending on functional groups present in target molecules to be synthesized, synthetic routes, including protection and deprotection steps, have to be designed for each molecule. To concisely synthesize functionalized molecules, chemical transformation methods that are not affected by functional groups presenting in starting materials are needed. It is a great advantage when the same reaction method can be used for the synthesis of a series of molecules bearing various functional groups without the need of product-specific protection and deprotection steps. In addition, it is desired that such reactions can be performed under safe, mild, and environmentally benign conditions. We address these points in our research as we design and develop catalysts and chemical transformation methods. By using organic molecules as catalysts, we concisely synthesize novel functionalized molecules including those that are often difficult to synthesize by traditional synthetic strategies. Our investigations into the chemical basis of the developed catalysts and chemical transformation methods further the understanding of the chemistry of organic molecules and their reactions.
2.1.1. Asymmetric construction of highly functionalized decalin derivatives
One of our recent achievements is the development of catalytic enantioselective formal (4+2) cycloaddition reactions of dihydropyran derivatives with cyclohexane-1,3-diones that afford functionalized decalins through aldol-aldol annulation (Scheme 1) (Chouthaiwale, Aher, and Tanaka, Angew. Chem. Int. Ed. 2018, 57, 13298). The decalin ring system is found in diterpenes, diterpenoids, steroids, and other bioactive molecules, so the development of methods for the synthesis of functionalized decalin derivatives is of interest in drug discovery and related research. Our strategy enabled the construction of polyfunctionalized decalins bearing five to six chiral carbon centers with high diastereo- and enantioselectivities from achiral molecules in a single transformation that formed two C-C bonds.

Scheme 1
Key factors of the success of the reactions include the use of the dihydropyran derivatives as the starting materials, which are obtained from pyruvates and aldehydes (Chouthaiwale and Tanaka, Chem. Commun. 2014, 50, 14881; Chouthaiwale, Lapointe, and Tanaka, Heterocycles 2017, 95, 587; see also section 2.1.2.). The starting material dihydropyran derivatives retain the a-keto ester group of pyruvates and are C2 symmetric molecules, allowing direct enantioselective transformations to provide the products in high yields (kinetic resolutions result in yields no higher than 50%).
During the aldol-aldol annulation cascade reaction, no accumulation of the aldol intermediate was detected; the product was detected at the initial stages of the reaction. In separate experiments, the product was stable under the reaction conditions; no changes in the enantiomeric excess were detected, and very little decomposition to the starting materials and/or to the aldol intermediate was observed. Based on these results, we suggest that the rate-limiting step is the second aldol step (i.e., the aldol annulation step). We are currently investigating the detail mechanisms of the reactions.
The utility of the reaction was demonstrated by transformations of the product to various decalin derivatives (Scheme 2). We are studying to improve and expand our strategy to enable the construction of other types of highly enantiomerically enriched, highly functionalized molecules.
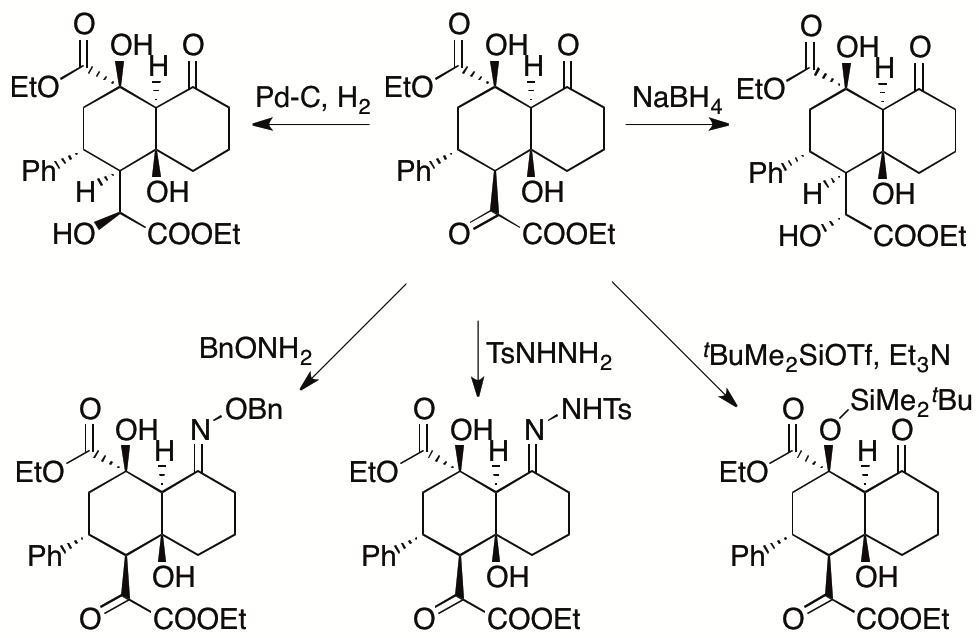
Scheme 2
2.1.2. Reactions of pyruvates to synthesize various functionalized molecules
Pyruvates can act as nucleophiles and electrophiles and thus are expected to be useful synthons. However, the dual reactivities of pyruvates are difficult to control. We have recently developed concise cascade reactions of pyruvates to provide various functionalized dihydropyrans in one pot under mild conditions (Scheme 3a) (Chouthaiwale and Tanaka, Chem. Commun. 2014, 50, 14881; Chouthaiwale, Lapointe, and Tanaka, Heterocycles 2017, 95, 587). Further, we have demonstrated that the product dihydropyrans are readily transformed to various molecules including amino group-substituted and fluoro group-substituted dihydropyrans, cyclohexane-derived amino acids, dihydrodiazepines, and pyridines (Scheme 3b).
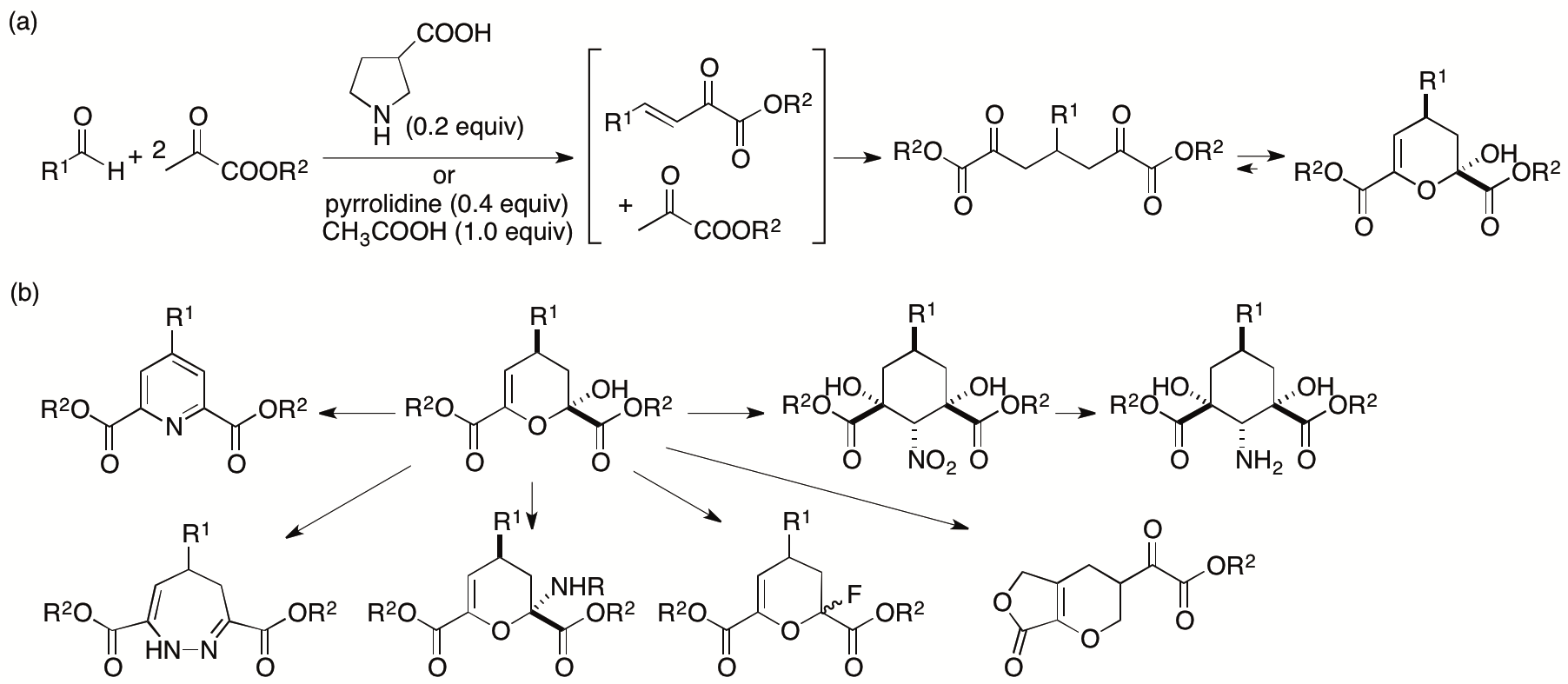
Scheme 3
As described in section 2.1.1., we have shown that the dihydropyrans are also used for the synthesis of functionalized decalin derivatives (Chouthaiwale, Aher, and Tanaka, Angew. Chem. Int. Ed. 2018, 57, 13298). We have also synthesized furopyrans and related derivatives from the dihydropyrans (Scheme 3b) (Chouthaiwale, Aher, and Tanaka, Heterocycles 2018, 97, 569).
We are further developing transformation methods that use the dihydropyrans obtained from pyruvates and aldehydes to concisely provide enantiomerically enriched, polyfunctionalized molecules by taming the reactivities of pyruvate derivatives. We are also developing catalyst systems that enable the reactions of pyruvates to provide functionalized molecules.
2.1.3. Asymmetric construction of spirooxindole polycycles
We have recently developed formal (4+1) cycloaddition and enantioselective Michael-Henry cascade reactions that provide spirooxindole polycycles (Scheme 4) (Huang, Sohail, Taniguchi, Monde, and Tanaka, Angew. Chem. Int. Ed. 2017, 56, 5853). Spiro[4,5]decanes and polycyclic compounds bearing spiro[4,5]decane systems are found in bioactive natural products. Functionalized molecules with these cyclic systems should be useful in drug discovery efforts. Our reactions provided spirooxindole polycyclic derivatives with seven stereogenic centers, including two all-carbon chiral quaternary centers and one tetrasubstituted chiral carbon center. Usually, such complex molecules are synthesized by multi step routes. Our strategies allow access to the complex functionalized molecules in highly enantiomerically enriched forms in two steps.
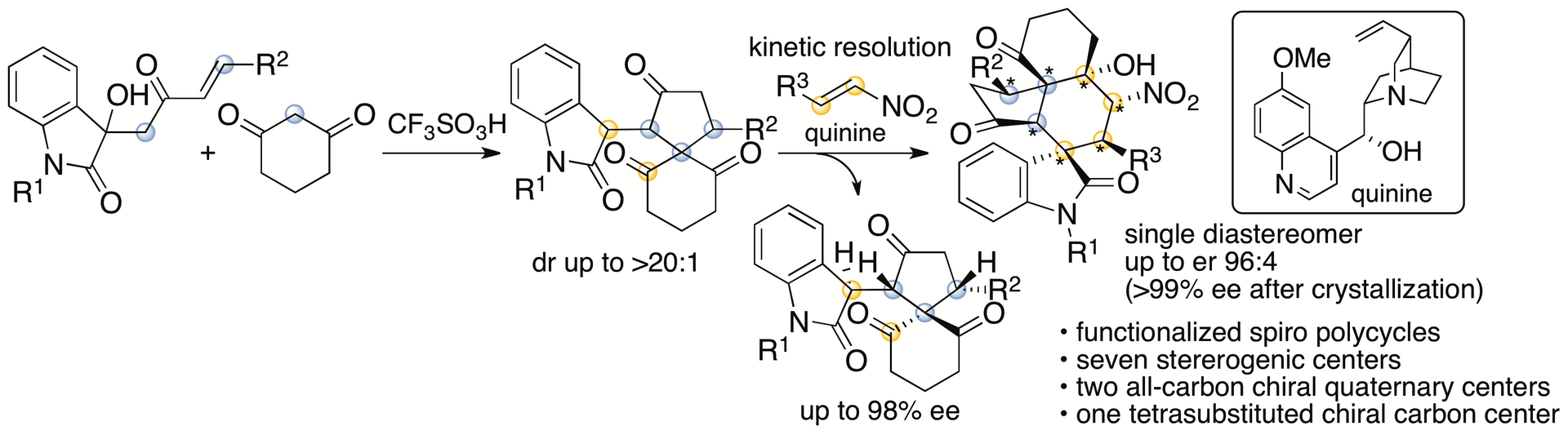
Scheme 4
We are currently investigating the mechanisms of the formal (4+1) cycloaddition reaction that provides the spiro[4,5]decane ring system. We are also investigating the mechanisms of the reactions of the spiro[4,5]decane derivatives to provide the polycyclic products and expanding the capabilities for the synthesis of functionalized molecules with polycyclic ring systems.
2.1.4. Asymmetric oxa-hetero-Diels-Alder reactions: Synthesis of spirooxindole tetrahydropyran derivatives
We recently reported catalytic asymmetric hetero-Diels-Alder reactions of enones with isatins (2,3-dioxyindoles or 2,3-dioxyindolins) that provide functionalized spirooxindole tetrahydropyranones with high diastereo- and enantioselectivities (Scheme 5) (Cui and Tanaka, Chem. Eur. J. 2013, 19, 6213). Novel amine-based catalyst systems composed of three-types of molecules (amine, acid, and thiourea) were developed to catalyze the reactions. The design and synthesis of single-molecule catalysts that provide all the required interactions for the catalysis and stereocontrol for designed reactions especially for new reactions are often difficult. We have demonstrated that the use of multicomponent catalyst systems provides a way to bypass the limitations in the access to efficient single component catalysts. We also elucidated the mechanism of the reactions and key factors for the high diastero- and enantioselectivities achieved by the three-component catalyst system (Cui, Chouthaiwale, Yin, and Tanaka, Asian J. Org. Chem. 2016, 5, 153).
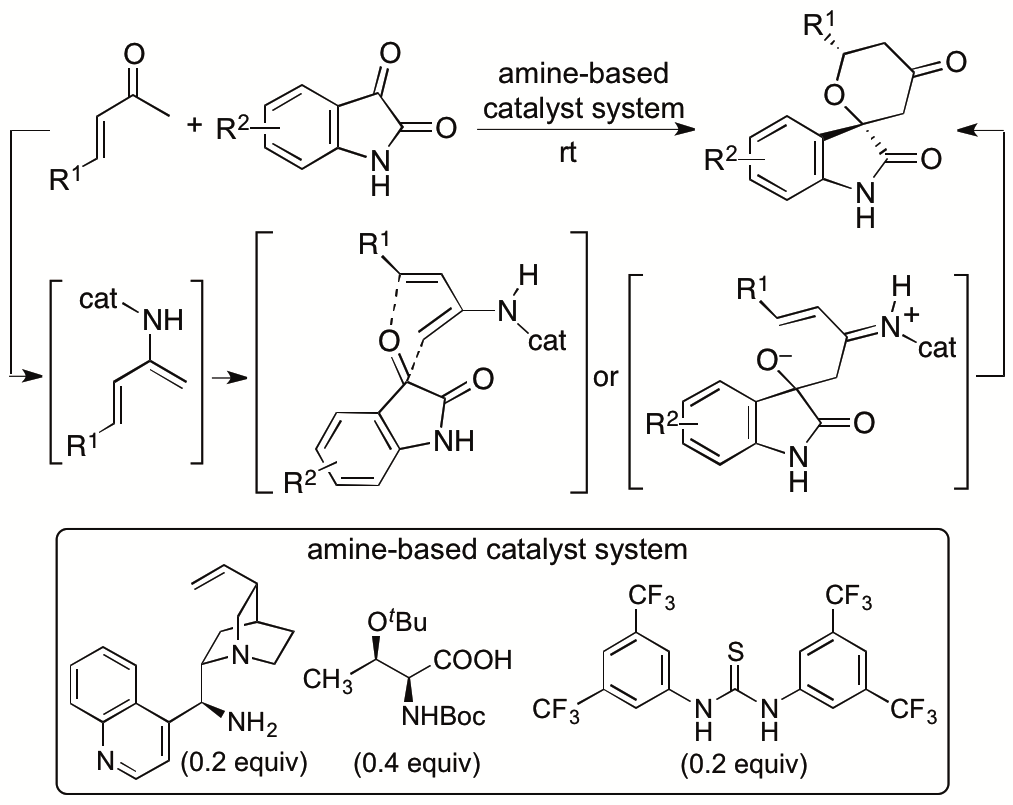
Scheme 5
Our developed hetero-Diels-Alder reaction method can provide the spirooxindole tetrahydropyranones as highly diastereo- and enantioselective forms. We are currently developing methods in which the minor product diastereomers in the previously developed reactions are the major diastereomers. We are also expanding the hetero-Diels-Alder reactions of enones beyond the use of the oxindole dienophiles to the use of various ketones and aldehydes as dienophiles.
2.1.5. Direct C-C bond-forming reactions of unprotected carbohydrates
Carbohydrate backbone-elongation reactions at anomeric carbons are important for the synthesis of higher carbon-backbone carbohydrates and of C-glycosides. Generally, reactions on carbohydrates require protection of hydroxy groups that are later deprotected. By considering atom- and step-economy, direct reactions on unprotected carbohydrates are preferable relative to the reactions requiring protection and deprotection steps and/or strategies requiring the synthesis of preactivated forms for the reactions. There are only limited numbers of non-enzymatic direct C-C bond forming reactions at the anomeric carbon of unprotected aldoses that are mainly present as cyclic hemiacetals. We have developed direct C-glycosidation reactions of unprotected aldopyranoses with ketones including the reactions of di- and trisaccharides (Scheme 6) (Johnson, Bagdi, and Tanaka, J. Org. Chem. 2018, 83, 4581; Enukonda, Johnson, and Tanaka, Heterocycles, 2018, 97, 569). We are continuing to develop carbohydrate backbone-elongation reactions of unprotected carbohydrates.
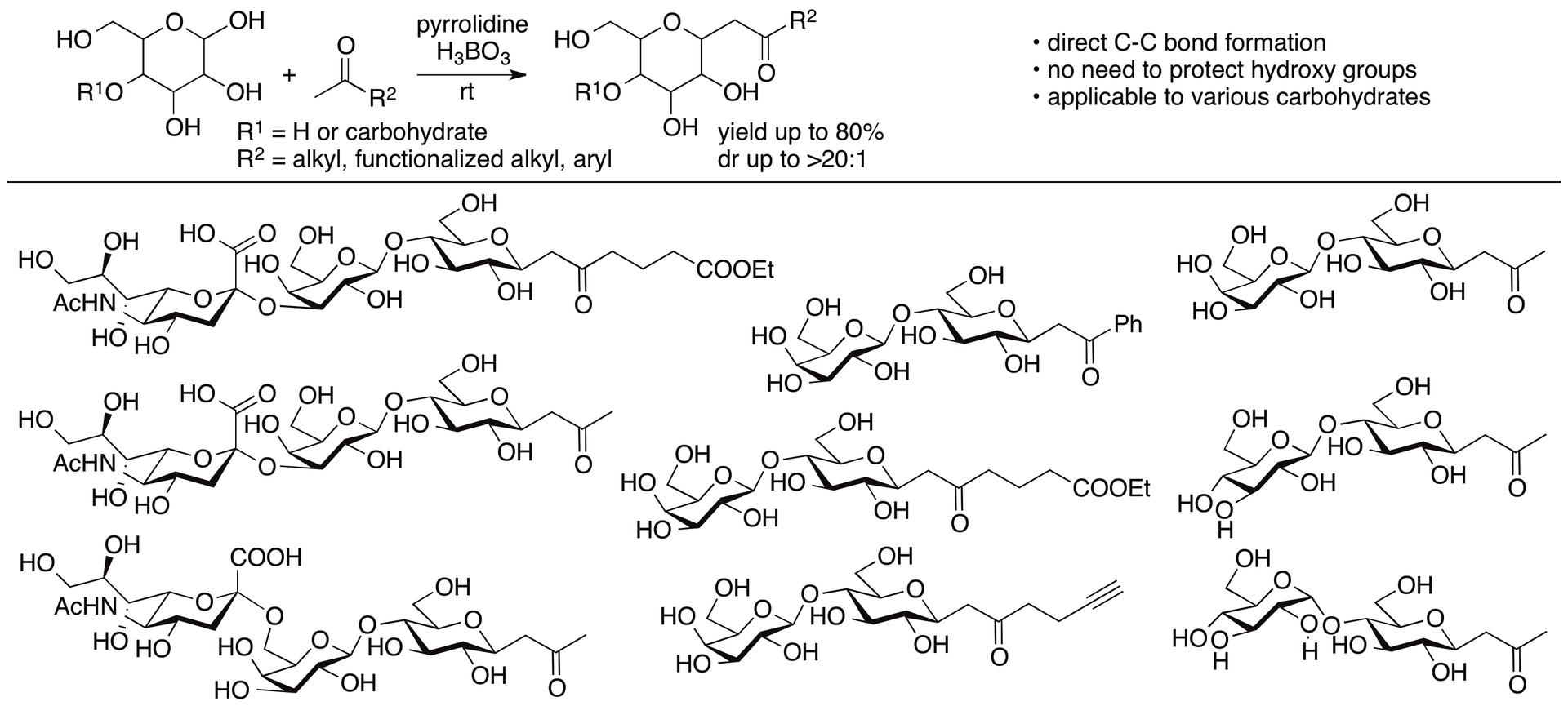
Scheme 6
2.1.6. Mannich reactions of carbohydrate derivatives with ketones
Polyoxy-functionalized piperidine derivatives are important as pharmaceuticals, probes, and their building blocks. Whereas various reaction methods for the synthesis of piperidine derivatives have been reported, most provide piperidines bearing only mono- and di-substitutions on the carbons of the piperidine rings. For the synthesis polyoxy-functionalized piperidines, strategies that are different from those used for the synthesis of simple piperidines are required. We have developed Mannich reactions of sugar derivatives with ketones that afford polyoxy-substituted piperidine derivatives bearing ketone groups (Scheme 7) (Maram and Tanaka, Org. Lett. 2019, 21, 1165). In our strategy, benzylamine used for the formation of the iminium ion in situ also activates the ketones via the formation of the enamines or the enolates. We have also identified conditions that lead to the formation of piperidine derivatives rather than tetrahydropyran derivatives.
The utility of this reaction method was further demonstrated by transformations of the products. For example, the piperidine derivatives obtained by this method were readily transformed to N-containing bicyclic derivatives, such as quinolizine derivatives.
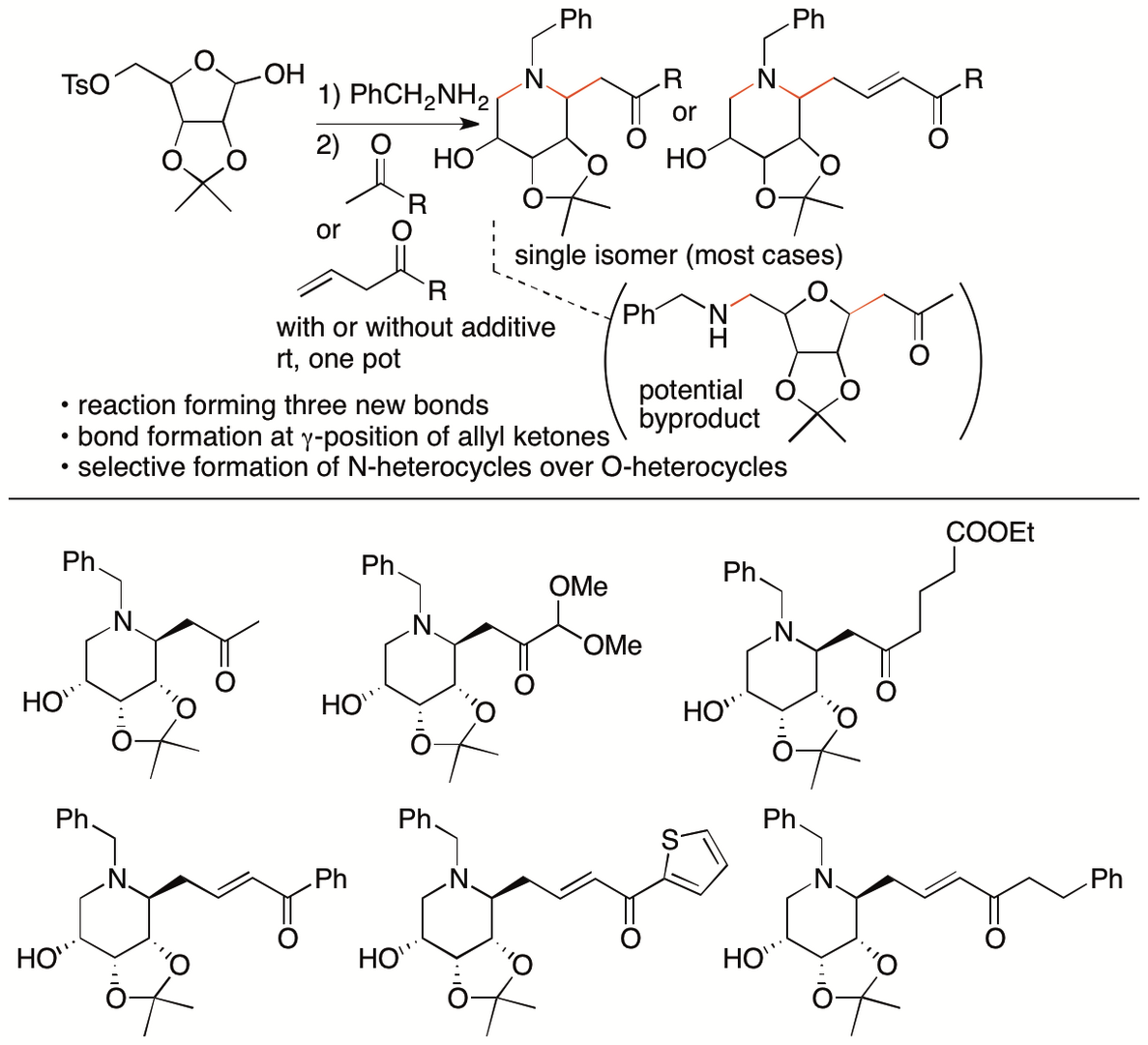
Scheme 7
2.1.7. γ-Position selective aldol and Mannich reactions of β-keto esters
In most aldol and Mannich reactions of β-keto esters in which the β-keto esters are used as nucleophiles, the bond formation occurs at the α-position of the β-keto esters. We have recently developed γ-selective aldol reactions of β-keto esters with aryl trifluoromethyl ketones catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (Scheme 8) (Zhang and Tanaka, Adv. Synth. Catal. 2015, 357, 3458). Using the obtained enantiomerically pure forms of the γ-aldol products derived from β-keto esters as enantiomer-discriminating agents, we have shown that enantiomers of chiral primary amines are concisely detected by 1H NMR through the formation of the enamines (Zhang, Chuang, Cao, Krishna, Shilpa, and Tanaka, Tetrahedron Letter, 2018, 59, 2248).
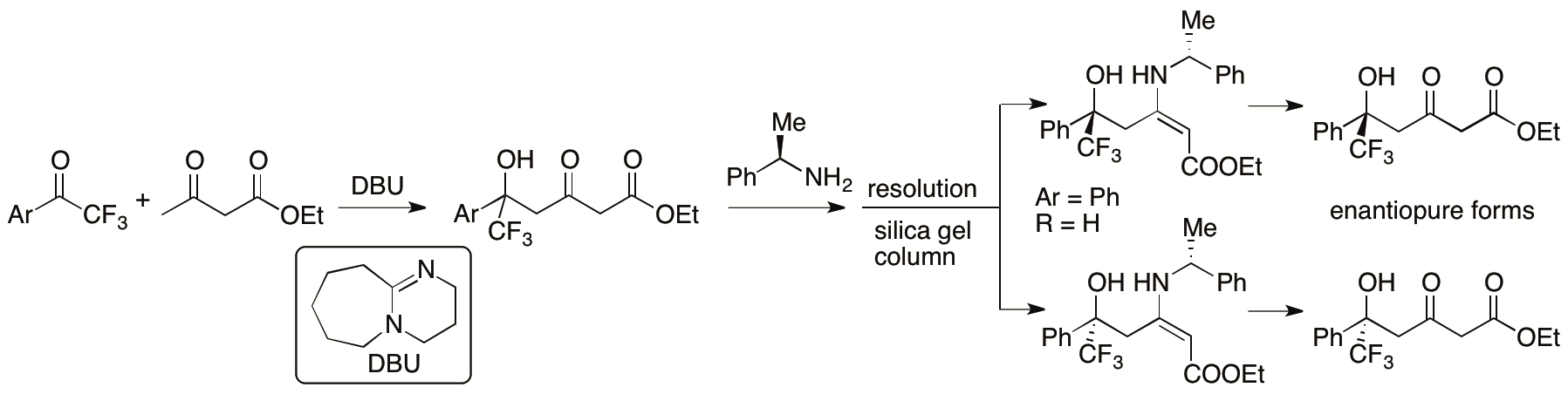
Scheme 8
To understand the unusual regioselectivity of the DBU-catalyzed aldol reactions of β-keto esters, deuteration experiments were performed (Zhang and Tanaka, Org. Lett. 2017, 19, 3803). We have found that the use of catalytic amount of DBU can enolize the β-keto ester at the γ-position in addition to the α-position. We are further investigating the mechanisms of the DBU-catalyzed γ-selective aldol reactions of β-keto esters. We are also working to expand the γ-selective reactions of β-keto esters to Mannich reactions and other reactions. We are also designing and synthesizing DBU-inspired chiral versions of catalysts to perform enantioselective aldol reactions and related reactions.
2.1.8. Asymmetric Michael reactions: Synthesis of pyrrolidine-3-carboxylic acid derivatives
Pyrrolidine-3-carboxylic acid (β-proline) derivatives and β2-amino acids are important molecules as bioactives, catalysts for chemical transformations, and their building blocks. To concisely synthesize these compounds, we have developed organocatalytic enantioselective Michael reactions of 4-alkyl-substituted-4-oxo-2-enoates with nitroalkanes (Scheme 9) (Yin, Garifullina, and Tanaka, Org. Biomol. Chem. 2017, 15, 6089; Yin, Garifullina, and Tanaka, Org. Biomol. Chem. 2018, 16, 3052). Using the developed method, highly enantiomerically enriched 5-methylpyrrolidine-3-carboxylic acid was synthesized in two steps from easily accessible starting materials.
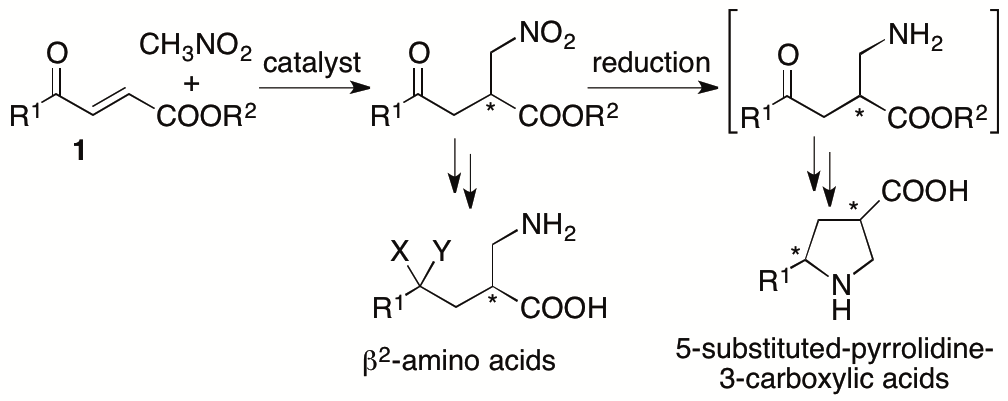
Scheme 9
2.2. Fluorescence-based reaction monitoring systems
We have been developing concise fluorescence-based assay methods to monitor bond-forming and bond-breaking reaction progress on a small scale to facilitate the development of catalysts and catalyzed chemical transformation methods. Use of fluorogenic substrates provides a straightforward method of reaction monitoring because reaction progress is directly observed as an increase in fluorescence.
For example, we have developed fluorogenic aldehydes based on a diarylacetylene core structure and fluorescence-based assay systems for aldol reactions using the fluorogenic aldehydes (Katsuyama, Chouthaiwale, Akama, Cui & Tanaka, Tetrahedron Lett. 2014, 55, 74). We are continuing to develop fluorogenic substrates and fluorescence-based assay systems with improved features.
2.3. Development of bioconjugation systems
Protein labeling methods are required for the synthesis of antibody-drug conjugates and other protein conjugates; these molecules are important as therapeutics and as detection devices for molecules of interest. Conjugation reactions are also needed to create multifunctional molecules. We are developing efficient protein labeling systems and molecules with desired reactivities that can be used for protein labeling reactions at targeted sites.
2.4. Search of biofunctional molecules
As described above, we have synthesized various functionalized molecules. In collaboration with researchers whose expertise is in biology and screening for biofunctional molecules, we have been searching new biofunctional molecules and drug leads. The collaborations include:
Professor Dr. Hiroshi Tomoda, Kitasato University
We are also working to expand the chemical space of molecules that we can synthesize and search for biofunctionl molecules by combining organic synthesis with microbial transformations in collaboration with Professor Dr. Katsuhiro Ueda, University of the Ryukyus.
3. Publications
3.1. Journals
- Yin, F.; Garifullina, A.; Tanaka, F. Correction: Synthesis of pyrrolidine-3-carboxylic acid derivatives via asymmetric Michael addition reactions of carboxylate-substituted enones. Organic & Biomolecular Chemistry 16, 3052-3053 (2018), doi: 10.1039/c8ob90051e.
- Chouthaiwale, P. V.; Aher, R. D.; Tanaka, F. Reactions of pyruvate-derived dihydropyrans with formaldehyde: synthesis of functionalized furopyrans and related products. Heterocycles 97, 569-573 (2018), doi: 10.3987/COM-18-S(T)29.
- Johnson, S.; Bagdi, A. K.; Tanaka, F. C-Glycosidation of unprotected di- and trisaccharide aldopyranoses with ketones using pyrrolidine-boric acid catalysis. The Journal of Organic Chemistry, 83, 4581-4597 (2018), doi: 10.1021/acs.joc.8b00340.
- Zhang, D.; Chuang, P.-S.; Cao, D.; Krishna, Y.; Shilpa, K.; Tanaka, F. Detection of enantiomers of chiral primary amines by 1H NMR analysis via enamine formation with an enantiopure g-position aldol product of a b-keto ester. Tetrahedron Letters, 59, 2248-2250 (2018), doi: 10.1016/j.tetlet.2018.04.079.
- Enukonda, J.; Johnson, S.; Tanaka, F. C-Glycosidation of unprotected aldopentoses with ketones using proline-triethylamine as catalyst. Heterocycles 99, in press, doi: 10.3987/COM-18-S(F)13.
- Chouthaiwale, P. V.; Aher, R. D.; Tanaka, F. Catalytic enantioselective formal (4+2) cycloaddition by aldol-aldol annulation of pyruvate derivatives with cyclohexane-1,3-diones to afford functionalized decalins. Angewandte Chemie Internal Edition, 57, 13298-13301 (2018), doi: 10.1002/anie.201808219. Angewandte Chemie, 130, 13482-13485 (2018), doi: 10.1002/ange.201808219.
- Maram, L.; Tanaka, F. Mannich reactions of carbohydrate derivatives with ketones to afford polyoxy-functionalized piperidines. Organic Letters 21, 1165-1169 (2019), doi: 10.1021/acs.orglett.9b00105.
3.2. Oral and Poster Presentations
- Tanaka, F. Generation of ketone nucleophiles and their reactions: Activation of substrates and control of reactions using organic molecules, in the class of Division of Chemistry, Graduate School of Science, Kyoto University, Kyoto, Japan, 2018.06.05. (invited, oral)
- Sohail, M.; Huang, J.-R.; Tanaka, F. Synthesis of enantiomerically enriched spiro[4,5]decanes and spirooxindole polycycles by formal (4+1) cycloaddition and Michael Henry-cascade reaction, in 7th EuCheMS Chemistry Congress, Liverpool, UK, 2018.08.26-2018.08.30 (oral OO29) (international conference)
- Sohail, M.; Huang, J.-R.; Tanaka, F. Enantioselective synthesis of complex oxindole-functionalized spiro polycycles, in IKCOC-14: The 14th International Kyoto Conference on New Aspects of Organic Chemistry, Kyoto, 2018.11.12-2018.11.16 (poster, PA(D)-38) (international conference)
- Maram, L.; Tanaka, F. Synthesis of iminosugar derivatives via Mannich reactions of carbohydrate derivatives with ketones, in IKCOC-14: The 14th International Kyoto Conference on New Aspects of Organic Chemistry, Kyoto, 2018.11.12-2018.11.16 (poster, PA(D)-40) (international conference)
- Krishna, Y.; Tanaka, F. Catalytic intramolecular vinylogous Mannich reaction of hydroxylactam-enals: Direct access to bicyclic izidine derivatives, in IKCOC-14: The 14th International Kyoto Conference on New Aspects of Organic Chemistry, Kyoto, 2018.11.12-2018.11.16 (poster, PB(C)-41) (international conference)
- Bethi, V.; Tanaka, F. Organocatalytic regioselective Mannich reactions of β-keto esters at the γ-position, in The 11th Symposium on Organocatalysis, Tokyo, 2018.12.03-2018.12.04 (poster, P25)
- Garg, Y.; Tanaka, F. Effects of metal salts in asymmetric aldol and Mannich reactions catalyzed by amino acids, in The 11th Symposium on Organocatalysis, Tokyo, 2018.12.03-2018.12.04 (poster, P26)
- Krishna, Y.; Tanaka, F. Catalytic intramolecular vinylogous Mannich reactions of hydroxylactam-enals: A route to bicyclic N-heterocycles, in The 139th Annual Meeting of the Pharmaceutical Society of Japan, Sendai, Japan (2019), 2019.03.20-2018.03.23. (poster, 21PO-am029)
- Maram, L.; Tanaka, F. Synthesis of piperidine derivatives via umpolung reactions of imines generated from carbohydrate derivatives, in The 139th Annual Meeting of the Pharmaceutical Society of Japan, Sendai, Japan (2019), 2019.03.20-2018.03.23. (poster, 21PO-pm020)
- Aher, R.; Chouthaiwale, P.; Tanaka, F. Organocatalytic enantioselective synthesis of functionalized decalins via desymmetrization of substituted dihydropyrans and 1,3-diketones, in the 254th ACS National Meeting, Orlando, Florida, 2019.03.31-2019.04.04. (oral, ORGN 523) (international conference)
4. Intellectual Property Rights
- Cui, H.; Tanaka, F. NOVEL SPIROOXINDOLE DERIVATIVE AND PROCESS FOR PRODUCING THE SAME, EP2888268 (Switzerland and United Kingdom, patent, granted on 2018.08.22), 13846040.7 (Germany, patent, granted on 2018.08.22).
- Zhang, D.; Tanaka, F. 5-SUBSTITUTED-5-HYDROXY-5-ARYL-3-OXO-PENTANOATE DERIVATIVES AND THEIR ENANTIOPURE FORMS, 10125074 (United States, patent, granted on 2018.11.13).
5. Meetings and Events
5.1. Seminars Hosted
- Date: April 18, 2018
- Venue: OIST campus
- Speaker: Professor Dr. Minoru Isobe, Nagoya University, Japan
- Title: Marine Natural Products---Synthesis of Ciguatoxin and Mechanism of Symplectoteuthis Bioluminescence
- Date: May 18, 2018
- Venue: OIST campus
- Speaker: Professor Dr. Nobutaka Numoto, Tokyo Medical and Dental University, Japan
- Title: Molecular recognition mechanism of proteins revealed by X-ray crystallography
- Date: October 11, 2018
- Venue: OIST campus
- Speaker: Professor Dr. Koichi Fukase, Osaka University, Japan
- Title: Synthetic Studies of Immunostimulating Glycoconjugates toward Cancer Immunotherapies
- Date: October 11, 2018
- Venue: OIST campus
- Speaker: Professor Dr. Antonio Molinaro, University of Napoli Federico II, Napoli, Italy
- Title: Microbial cell wall glycoconjugates and elicitation/suppression of eukaryotic innate immunity
- Date: November 26, 2018
- Venue: OIST campus
- Speaker: Professor Dr. Norio Shibata, Nagoya Institute of Technology, Japan
- Title: Synthesis of Pentafluoro- and Tetrafluoro-λ6-sulfane Derivatives
- Date: November 26, 2018
- Venue: OIST campus
- Speaker: Professor Dr. David O’Hagan, University of St Andrews, UK
- Title: Isolation and applications of the fluorinase (a fluorination enzyme)
- Date: March 8, 2019
- Venue: OIST campus
- Speaker: Professor Dr. Ikuo Fujii, Osaka Prefecture University, Japan
- Title: Peptide-Drug Conjugates: Intracellular Drug Delivery by the Anti-VEGF Helix-loop-helix Peptides Inducing Receptor-mediated Endocytosis



