FY2014 Annual Report
Chemistry and Chemical Bioengineering Unit
Professor: Dr. Fujie Tanaka, Associate Professor
Abstract
The ability to design and synthesize organic molecules constitutes the foundation of that underlies basic research as well as applied science. It is also essential for the development of pharmaceuticals and biofunctional molecules. This unit develops new, efficient, concise, and safe chemical transformation methods and strategies for constructing small molecules bearing functional groups and/or chiral centers that are relevant to the creation of biofunctional molecules. We mainly develop reaction strategies that use small organic molecules as enzyme-like catalysts. With the use of organic molecules as catalysts, we minimize the need for protection and deprotection steps that are usually required for the synthesis of functionalized molecules. When a reaction method does not affect functional groups that are not at the reaction site, the reaction method can be used for the synthesis of a series of molecules bearing various functional groups. That is, a series of molecules of interest may be synthesized using the same method in a short route, providing advantages for the synthesis of biofunctional candidate molecules. We also investigate the chemical bases of the reactions to understand the mechanisms of the catalysis and molecular interactions provided by organic molecules. By taking advantage of the use of features of our developing molecules, we also develop strategies and methods to conjugate proteins and peptides with other molecules. The molecules that we have synthesized are screened in various functional assays in collaboration with other research groups. The research undertaken by this unit advances the chemistry of catalysis and of molecular synthesis. The studies by this unit accelerate the creation of molecules used in biomedical research and contribute to the development of new therapeutics, therapeutic strategies, and diagnostics.
1. Staff
- Dr. Pandurang V. Chouthaiwale
- Dr. Hai-Lei Cui
- Dr. Jithender Enukonda
- Dr. Ji-Rong Huang
- Dr. Sherida Johnson
- Dr. Feng Yin
- Mr. Jyunsuke Machida
- Ms. Maria Rosa Mendoza Quijano
- Mr. Dongxin Zhang, Graduate Student
- Ms. Nidhi Pant, Research Intern
- Ms. Diana Marcela Soto Martinez, Research Intern
- Ms. Shiho Chinen, Research Administrator
- Ms. Tomo Kohatsu, Research Administrator
2. Activities and Findings
2.1. Development of new chemical transformation methods and synthesis of functionalized molecules
We have been developing small organic molecule catalysts (organocatalysts) and organocatalytic molecular transformation methods useful for the synthesis of functionalized molecules under mild conditions in short routes. We also investigate chemical basis of the catalysis and the chemical transformations to understand the mechanisms of the catalysis and molecular interactions provided by organic molecules to further the creation of useful molecules.
Traditional synthetic methods often require high or very low temperature and/or absolute conditions. In addition, functional groups on substrate molecules must be protected prior reactions. That is, depending on functional groups present in target molecules to be synthesized, synthetic routes, including protection and deprotection steps, have to be designed for each molecule. To concisely synthesize functionalized molecules, chemical transformation methods that are not affected by functional groups presenting in starting materials are needed. It is a great advantage when the same reaction method can be used for the synthesis of a series of molecules bearing various functional groups without the need of product-specific protection and deprotection steps. In addition, it is desired that such reactions can be performed under safe, mild, and environmentally benign conditions. We address these points in our research as we design and develop catalysts and chemical transformation methods. By using organic molecules as catalysts, we concisely synthesize novel functionalized molecules and groups of functionalized molecules that are often difficult to synthesize by traditional synthetic strategies. Our studies provide molecules that are screened for bioactive candidates and contribute to create new functional molecules. Our investigation on the chemical basis of the developed catalysts and chemical transformation methods further the understanding of the chemistry of organic molecules and their reactions.
2.1.1. Asymmetric hetero-Diels-Alder reactions
One of the recent achievements of this unit is the development of catalytic asymmetric hetero-Diels-Alder reactions that provide functionalized spirooxindole tetrahydropyranones with high diastereo- and enantioselectivities (Scheme 1) (Cui & Tanaka, Chem. Eur. J. 2013, 19, 6213). Novel amine-based catalyst systems composed of three-types of molecules (amine, acid, and thiourea) have been developed to catalyze the reactions of enones. Substituted tetrahydropyranones are important for the syntheses of bioactive molecules, and the developed reactions provide concise, atom-economical routes to these compounds. The products have ketone and oxindole amide groups. Using these groups as further reaction sites, various spirooxindole derivatives can also be synthesized. As both the spirooxindole derivatives and the tetrahydropyranes are important bioactive molecules, the synthesized molecules themselves are of interest.
Scheme 1 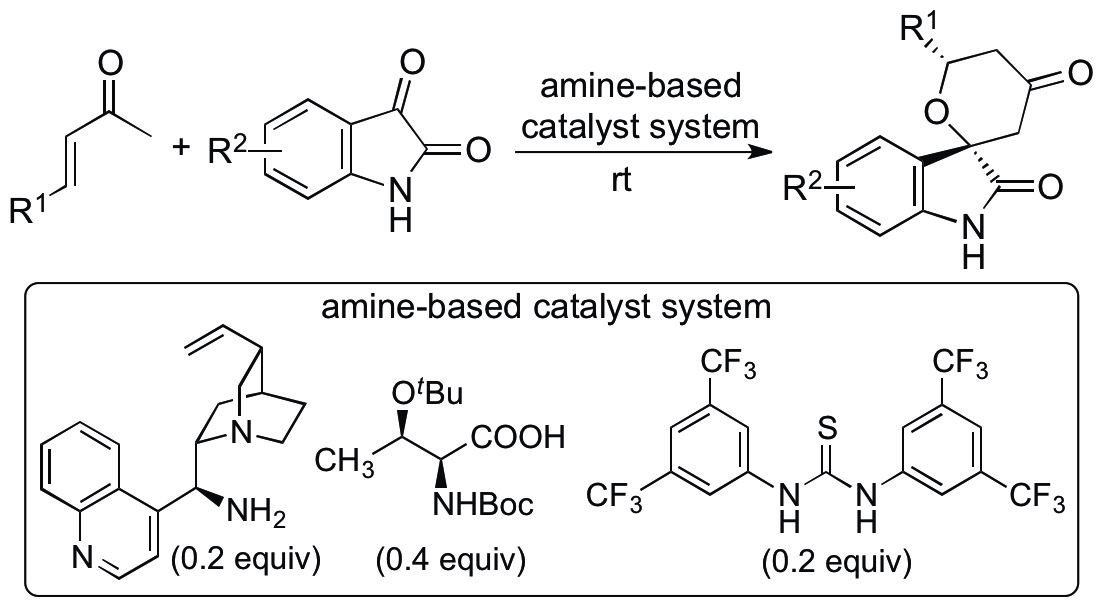
Because the hetero-Diels-Alder reactions that directly use enones as starting materials without the requirement of pre-formation of dienes for the hetero-Diels-Alder reactions are significant, we have been investigating the mechanism of the reactions and key factors for the high diastero- and enantioselectivities. Our reaction-based investigation indicated that the products formed via a kinetically controlled [4+2] cycloaddition of the in situ-generated enamine of the enone as the diene and isatin as the dienophile (Cui, Chouthaiwale, Yin & Tanaka, Asian J. Org. Chem. in press, DOI: 10.1002/ajoc.201500412).
We have also been studying to expand the oxa-Diels-Alder reactions beyond the use of the oxindole dienophiles to the use of various ketones and aldehydes as dienophiles, and to aza-Diels-Alder reactions that use imines as dienophile reactants.
2.1.2. Synthesis of furanose spirooxindoles via fast aldol reactions
Spirooxindoles are important as they are often found in bioactive molecules as described in section 2.1.1. Because furanose units are present in biomolecules, furanose spirooxindoles may be useful as biofunctional molecule candidates. We have designed and synthesized furanose spirooxindoles (Scheme 2) (Zhang, Johnson, Cui & Tanaka, Asian J. Org. Chem. 2014, 3, 391). The first step was an aldol reaction of a pyruvic aldehyde derivative 1,1-dimethoxypropan-2-one with an isatin; the following reduction of the ketone carbonyl group generated the furanose spiro system.
Scheme 2 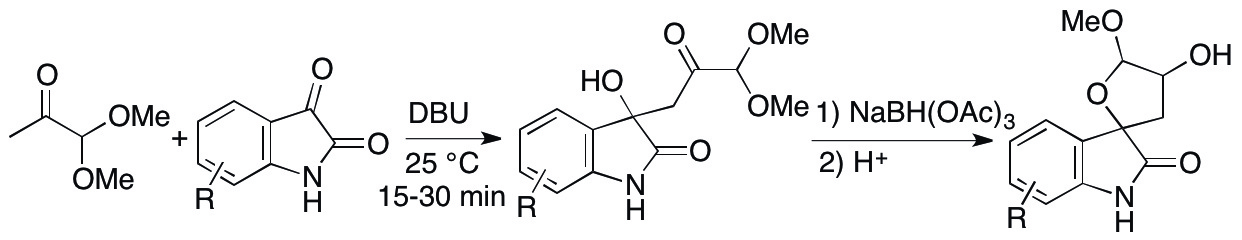
Whereas the pyruvic aldehyde derivative is a useful synthon and has been used in many types of reactions including aldol reactions, previously reported aldol reactions of this ketone under mild conditions were relatively slow; the reaction time was often several hours to several days to give the aldol products in reasonable yields. We searched for catalysts and conditions for fast aldol reactions of the aldehyde with isatin and found that DBU is a suitable catalyst. With DBU as catalyst, the aldol reaction products of various substituted isatins were obtained in good to high yields within 15 to 30 min. Subsequently, the aldol products were transformed to the furanose spirooxindoles.
2.1.3. DBU-catalyzed aldol reactions to construct trifluoromethyl-substituted tertiary alcohols
Based on the success of the fast aldol reaction catalyzed by DBU described above, we have expanded the DBU-catalysis to aldol reactions of aryl trifluoromethyl ketones with various donor substrates to give trifluoromethyl-substituted tertiary alcohols (Zhang & Tanaka, Adv. Synth. Catal. 2015, in press, DOI: 10.1002/adsc.201500497). In the DBU-catalyzed aldol reactions, the C-C bonds formed in perfect regioselectivities at the methyl groups of alkyl methyl ketones, at the γ-positions of β-keto esters, and at the methyl groups of the β-methyl-substituted cyclic enones (Scheme 3). For the aldol products from the β-keto esters, enantiomerically pure forms were obtained by the resolution of the enamines of the aldol products with a homochiral amine. With DBU as the catalyst, construction of tetra-substituted alcohols bearing the trifluoromethyl group, which is difficult and/or usually requires severe conditions to synthesize, was concisely achieved under mild conditions. In addition, concise resolution method was established to provide access to pure enantiomers of the products from the reactions of β-keto esters. We are also designing and synthesizing DBU-inspired chiral versions of catalysts to perform enantioselective aldol reactions and related reactions.
Scheme 3 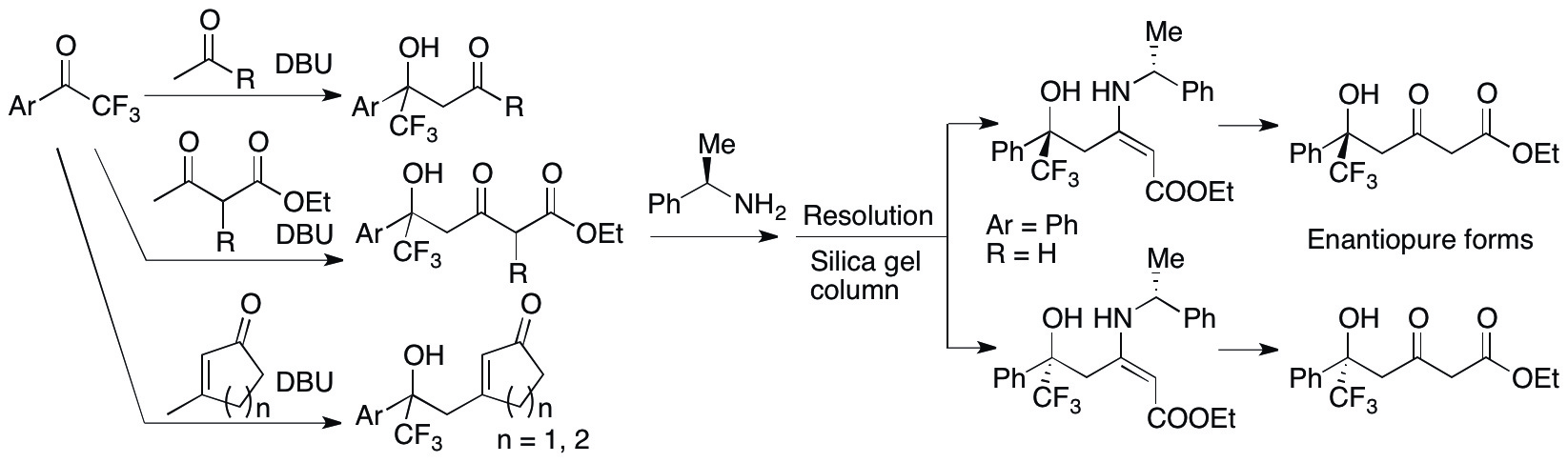
2.1.4. Aldol reactions of 1,2-diketones to afford furanose derivatives
As described in the section 2.1.2., furanose units are present in biomolecules. We have developed aldol methods using 1,2-diketones as starting materials to access substituted furanose derivatives concisely (Jithender, Katsuyama, Zhang, Johnson & Tanaka, Tetrahedron Lett. 2015, 56, 735). The 1,2-diketones are expected to be useful synthons because they act as both nucleophiles (such as enolates and enamines) and electrophiles. However, control of the reactivity of 1,2-diketones is difficult; reactions with 1,2-diketones may result in the formation of undesired polymerized products. We have developed the first examples of aldol reactions of acyclic 1,2-diketones in which the 1,2-diketones act as nucleophiles (Scheme 4). In our methods, depending on the catalyst system used, the C-C bond formation regioselectively occurs at either at the methyl group carbon or at the substituted methylene carbon.
Scheme 4 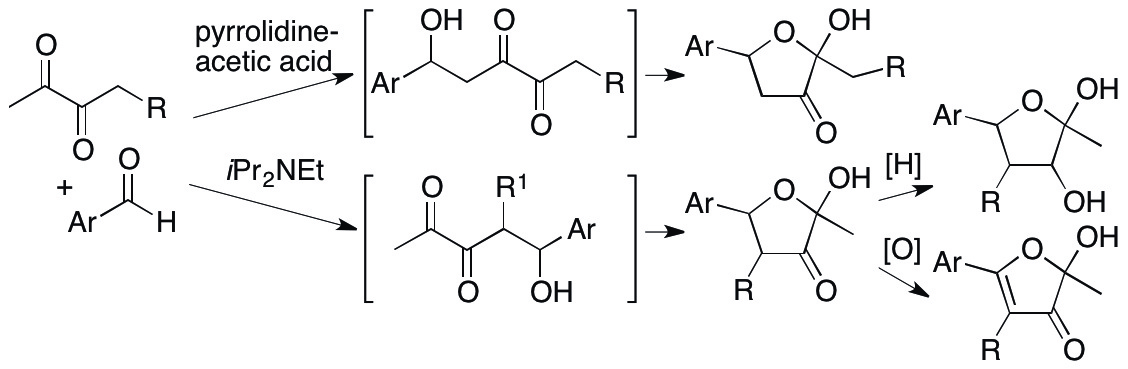
2.1.5. Cascade reactions of pyruvates with aldehydes to synthesize dihydropyran derivatives
Pyruvates can act as nucleophiles and electrophiles and thus are expected useful synthons. However, like reactions of 1,2-diketones described in 2.1.4, the dual reactivities of pyruvates are difficult to control. We have developed concise cascade reactions of pyruvates to provide various functionalized dihydropyrans in one pot under mild conditions (Scheme 5) (Chouthaiwale & Tanaka, Chem. Commun. 2014, 50, 14881). Further, we have demonstrated that the product dihydropyrans are readily transformed to various molecules including amino group-substituted and fluoro group-substituted ddihydropyrans, cyclohexane-derived amino acids, dihydrodiazepines, and pyridines. These transformations can be performed under mild conditions and the products are relevant to the search of biofunctional molecules.
Scheme 5 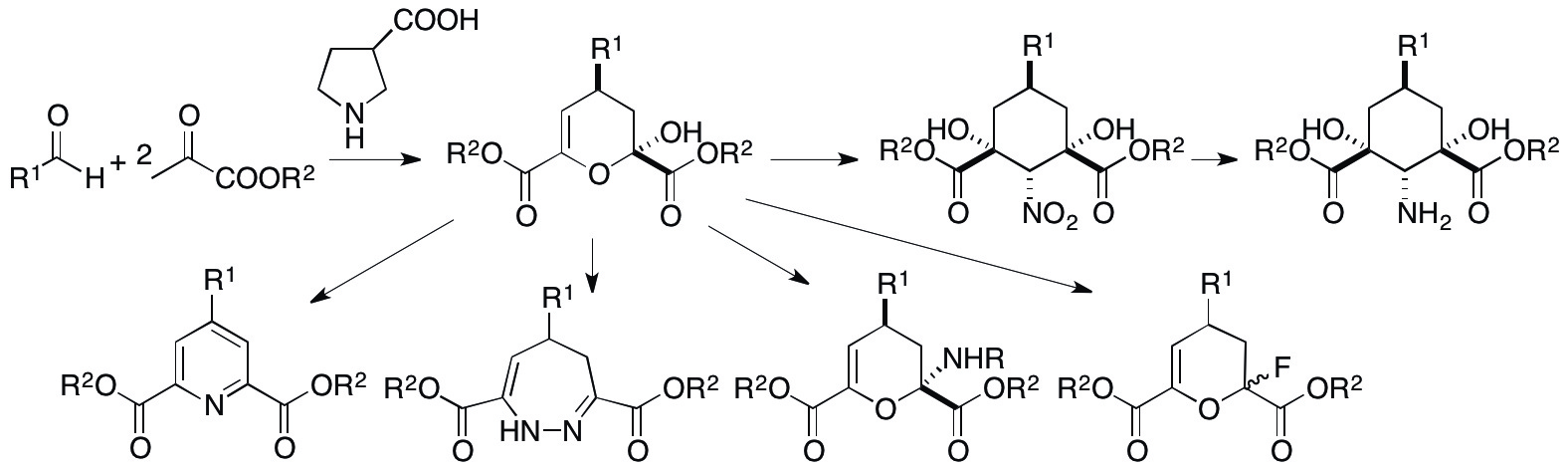
2.1.6. Direct C-C bond-forming reactions of unprotected carbohydrates
Carbohydrate backbone elongation reactions at anomeric carbons are important for the synthesis of higher carbon-backbone carbohydrates and C-glycosides. Generally, reactions on carbohydrates require protection of hydroxy groups that are later deprotected. If unprotected carbohydrates could be used as substrates, the methods would be atom- and step-economical. However, limited numbers of non-enzymatic direct C-C bond forming reactions at the anomeric carbon of unprotected aldoses, which are mainly present as cyclic hemiacetals, have been reported. We have been developing direct C-C bond-forming reactions of unprotected aldohexoses and aldopentoses.
2.2. Fluorescence-based reaction monitoring systems
This unit has also been developing concise fluorescence-based assay methods to monitor bond-forming and bond-breaking reaction progress on a small scale to facilitate the development of catalysts and catalyzed chemical transformation methods. Use of fluorogenic substrates provides a straightforward method of reaction monitoring because reaction progress is directly observed as an increase in fluorescence.
For example, we have developed fluorogenic aldehydes based on a diarylacetylene core structure and fluorescence-based assay systems for aldol reactions using the fluorogenic aldehydes (Scheme 6) (Katsuyama, Chouthaiwale, Akama, Cui & Tanaka, Tetrahedron Lett. 2014, 55, 74). We continue to develop fluorogenic substrates and fluorescence-based assay systems with improved features.
Scheme 6 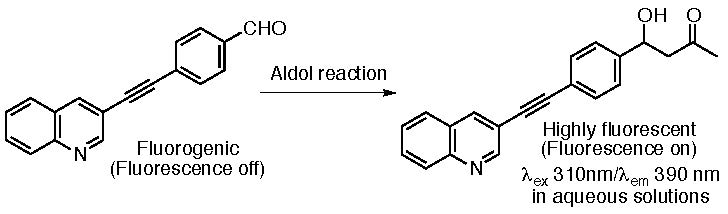
2.3. Development of bioconjugation systems
Protein labeling methods are required for the synthesis of antibody-drug conjugates and other protein conjugates; these molecules are important as therapeutics and as detection devices for molecules of interest. Conjugation reactions are also needed to create multifunctional molecules. We are developing efficient protein labeling systems and molecules with desired reactivities that can be used for protein labeling reactions at targeted sites.
2.4. Search of biofunctional molecules
As described above, we have synthesized various functionalized molecules. In collaborating with researchers whose expertise is in biology and screening for biofunctional molecules, we have been searching new biofunctional molecules and drug leads. The collaborations include:
• Professor Hiroshi Tomoda, Kitasato University
• Screening Committee of Anticancer Drugs supported by Grant-in-Aid for Scientific Research on Innovative Areas, Scientific Support Programs for Cancer Research, from The Ministry of Education, Culture, Sports, Science and Technology, Japan
• Lilly Open Innovation Drug Discovery
• Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”
3. Publications
3.1. Journals
- Katsuyama, I., Chouthaiwale, P. V., Akama, H., Cui, H.-L. & Tanaka, F. Fluorogenic probes for aldol reactions: Tuning of fluorescence using π-conjugation systems. Tetrahedron Letters 55, 74-78 (2014), doi: 10.1016/j.tetlet.2013.10.122.
- Mase, N., Ando, T., Shibagaki, F., Sugita, A., Narumi, T., Toda, M., Watanabe, N. & Tanaka, F. Fluorogenic aldehydes bearing arylethynyl groups: turn-on aldol reaction sensors for evaluation of organocatalysis in DMSO. Tetrahedron Letters 55, 1946-1948 (2014), doi: 10.1016/j.tetlet.2014.02.007.
- Zhang, D., Johnson, S., Cui, H.-L. & Tanaka, F. Synthesis of furanose spirooxindoles via 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU)-catalyzed aldol reactions of a pyruvic aldehyde derivative. Asian Journal of Organic Chemistry 3, 391-394 (2014), doi: 10.1002/ajoc.201400016.
- Cui, H.-L. & Tanaka, F. One-pot synthesis of polysubstituted 3-acylpyrroles by cooperative catalysis. Organic & Biomolecular Chemistry 12, 5822-5826 (2014), doi: 10.1039/c4ob01019a.
- Chouthaiwale, P. V. & Tanaka, F. Reactions of pyruvates: organocatalytic synthesis of functionalized dihydropyrans in one pot and further transformations to functionalized carbocycles and heterocycles. Chemical Communications 50, 14881-14884 (2014), doi: 10.1039/c4cc06035k.
- Jithender, E.; Katsuyama, I.; Zhang, D.; Johnson, S. & Tanaka, F. Aldol reactions of 1,2-diketones catalyzed by amines to afford furanose derivatives. Tetrahedron Letters 56, 735-738 (2015), doi: 10.1016/j.tetlet.2014.12.094.
- Zhang, D. & Tanaka, F. Aldol reactions of ketone donors with aryl trifluoromethyl ketone acceptors catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) for concise access to aryl- and trifluoromethyl-substituted tertiary alcohols. Advanced Synthsis & Catalysis, in press, doi: 10.1002/adsc.201500497.
- Cui, H.-L., Chouthaiwale, P. V.; Yin, F. & Tanaka, F. Reaction-based mechanistic investigations of asymmetric hetero-Diels-Alder reactions of enones with isatins catalyzed by amine-based three-component catalyst systems. Asian Journal of Organic Chemistry, in press, doi: 10.1002/ajoc.201500412.
3.2. Books and other one-time publications
- Tanaka, F. Development of efficient amine-based catalyst systems: Control of reactions of molecules that act as both nucleophiles and electrophiles. In MEXT Grant Advanced Molecular Transformations by Organocatalysts News Letter No. 39 (2015).
3.3. Oral and Poster Presentations
- Cui, H.-L. & Tanaka, F. Asymmetric hetero-Diels Alder reaction: Synthesis of spirooxindole-bearing tetrahydropyranones, in Screening Committee of Anticancer Drugs 3rd Symposium, Natural Products in the Academia-originated Development of Anticancer Drugs, Nago, Okinawa, Japan (2014), 2014.05.12.
- Tanaka, F. Synthesis of sugar derivatives for screening of bioactive molecules, in The 1nd Committee Meeting (H26), Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”, Naha, Okinawa, Japan (2014), 2014.06.09.
- Cui, H.-L. & Tanaka, F. Asymmetric formal oxa-Diels Alder reaction catalyzed by amine-based catalysts affording functionalized spirooxindole tetrahydropyrans, in The 14th Belgian Organic Synthesis Symposium, Louvain-la-Neuve, Belgium (2014), 2014.07.13-2014.07.18.
- Zhang, D. Johnson, S., Cui, H.-L. & Tanaka, F. Synthesis of Spirooxindole Furanose Derivatives via DBU-Catalyzed Aldol Reactions, in The 14th Belgian Organic Synthesis Symposium, Louvain-la-Neuve, Belgium (2014), 2014.07.13-2014.07.18.
- Chouthaiwale, P. V. & Tanaka, F. β-Proline-catalyzed reactions of pyruvates to synthesize functionalized molecules, in Advanced Molecular Transformations by Organocatalysts 2nd International Conference and 7th Symposium on Organocatalysis, Tokyo, Japan (2014), 2014.11.21-2014.11.22.
- Cui, H.-L. & Tanaka, F. Cooperative catalysis: synthesis of 3-acylpyrroles and 2,5-dihydropyrroles via aza-Michael-alkyne carbocyclization cascade from enones and propargylamines, in Symposium, Okinawa Intellectual Cluster Program, Naha, Okinawa, Japan (2014), 2014.12.18.
- Cui, H.-L. & Tanaka, F. Asymmetric formal oxa-Diels-Alder reaction catalyzed by amine-based catalysts affording functionalized spirooxindole tetrahydropyrans, in Symposium, Okinawa Intellectual Cluster Program, Naha, Okinawa, Japan (2014), 2014.12.18.
- Johnson, S. & Tanaka, F. Organocatalytic aldol reactions of C6 pyranoses to synthesize C9 sugars, in Symposium, Okinawa Intellectual Cluster Program, Naha, Okinawa, Japan (2014), 2014.12.18.
- Chouthaiwale, P. V. & Tanaka, F. β-Proline-catalyzed reactions of pyruvates to synthesize functionalized molecules, in Symposium, Okinawa Intellectual Cluster Program, Naha, Okinawa, Japan (2014), 2014.12.18.
- Zhang, D.; Johnson, S.; Cui, H.-L. & Tanaka, F. Synthesis of spirooxindole furanose derivatives via DBU-catalyzed aldol reactions, in Symposium, Okinawa Intellectual Cluster Program, Naha, Okinawa, Japan (2014), 2014.12.18.
- Tanaka, F. Synthesis of sugar derivatives for screening of bioactive molecules, in The 2nd Committee Meeting (H26), Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”, Naha, Okinawa, Japan (2015), 2015.01.09.
- Jithender, E.; Katsuyama, I.; Zhang, D.; Johnson, S. & Tanaka, F. Synthesis of furanose derivatives via amine-catalyzed aldol reactions of 1,2-diketones, in The 135th Annual Meeting of the Pharmaceutical Society of Japan, Kumamoto, Japan (2015), 2015.03.25-2014.03.28.
- Johnson, S. & Tanaka, F. Organocatalytic tandem aldol condensation-Michael reactions of C6 pyranoses to synthesize C-glycosides, in The 135th Annual Meeting of the Pharmaceutical Society of Japan, Kobe, Japan (2015), 2015.03.25-2015.03.28.
- Zhang, D. & Tanaka, F. Aldol reactions using aryltrifluoromethyl ketones as electrophiles catalyzed by 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) under solvent free mild conditions, in The 135th Annual Meeting of the Pharmaceutical Society of Japan, Kumamoto, Japan (2015), 2014.03.25-2015.03.28.
- Suwa, I.; Uchida, R.; Cui, H.-L.; Chouthaiwale, P. V.; Tanaka, F.; Katagiri, . & Tomoda, H. Study on BMP signaling inhibitors from synthetic compound libraries, in The 135th Annual Meeting of the Pharmaceutical Society of Japan, Kumamoto, Japan (2015), 2015.03.25-2015.03.28.
- Johnson, S. & Tanaka, F. Stereoselective synthesis of C-glycosides from unprotected C6 aldopyranoses via amine-catalyzed aldol condensation-oxa-Michael addition reactions, in The 8th Symposium on Organocatalysis and 5th Advanced Molecular Transformations by Organocatalysts Symposium, Naha, Japan (2015), 2015.05.10-2015.05.11.
- Tanaka, F. Molecular synthesis for the creation of new bioactive molecules, in The 43rd Japan Ulcer Society Meeting, Onna, Okinawa, Japan (2015), 2015.06.19-2015.06.20.
- Tanaka, F. Synthesis of sugar derivatives for screening of bioactive molecules, in The 2nd Committee Meeting (H27), Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”, Naha, Okinawa, Japan (2015), 2015.06.24.
- Zhang, D. & Tanaka, F. DBU-catalyzed regioselective aldol reactions to synthesize functionalized molecules with tetrasubstituted carbon centers, in The 38th Naito Conference The Chemistry of Organocatalysts, Sapporo, Japan (2015), 2015.07.06-2015.07.09.
- Tanaka, F. Synthesis of sugar derivatives for screening of bioactive molecules, in The 1st PI Meeting (H27), Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”, Naha, Okinawa, Japan (2015), 2015.09.15.
4. Intellectual Property Rights and Other Specific Achievements
- Chouthaiwale, P. V. & Tanaka, F. 4-Substituted pyridine-2,6-dicarboxylic acid derivatives and method of preparing same. US patent application, US 62/137,203 (filing date 2014.09.09).
- Tanaka, F. & Zhang, D. 5-Substituted-5-hydroxy-5-aryl-3-oxo-pentanoate derivatives and their enantiopure forms. Provisional US patent application, US 62/137,387 (filing date 2015.03.24).
- Tanaka, F. & Johnson, S. C-Glycoside derivatives. Provisional US patent application, US 62/137,380 (filing date 2015.03.24).
5. External Funding
1. Grant-in-Aid for Scientific Research on Innovative Areas “Advanced Molecular Transformations by Organocatalysts” from The Ministry of Education, Culture, Sports, Science and Technology (MEXT); Japan Society for the Promotion of Science (JSPS), Japan
Title: Development of efficient amine-based catalyst systems
PI: Tanaka, Fujie
April 2014 – March 2015
2. Grant-in-Aid for Scientific Research on Innovative Areas “Advanced Molecular Transformations by Organocatalysts” from The Ministry of Education, Culture, Sports, Science and Technology (MEXT); Japan Society for the Promotion of Science (JSPS), Japan
Title: Development of efficient amine-based catalyst systems
PI: Tanaka, Fujie
April 2015 – March 2016
3. Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”
Title: Synthesis of sugar derivatives for screening of bioactive molecules
PI: Tanaka, Fujie
April 2014 – March 2015
4. Okinawa Intellectual Cluster Program “Exploration Research on The Development of Pharmaceuticals and Their Candidates Using Bioresources and Networks in Okinawa”
Title: Synthesis of sugar derivatives for screening of bioactive molecules
PI: Tanaka, Fujie
April 2015 – February 2016
6. Meetings and Events
6.1. Seminar Hosted
6.1.1. Seminar 1
- Date: August 21, 2014
- Venue: OIST campus
- Speaker: Professor Dr. Keiji Maruoka, Department of Chemistry, Graduate School of Science, Kyoto University, Japan
- Title: Challenges in organocatalytic chemistry: Design of organoradical catalysts and their synthetic application
6.1.2. Seminar 2
- Date: September 29, 2014
- Venue: OIST campus
- Speaker: Professor Dr. Masahiro Terada, Department of Chemistry, Graduate School of Science, Tohoku University, Japan
- Title: Enantioselective catalysis by chiral Brønsted acids and bases
6.1.3. Seminar 3
- Date: October 6, 2014
- Venue: OIST campus
- Speaker: Professor Dr. Jun’ichi Kobayashi, Graduate School of Pharmaceutical Science, Hokkaido University, Japan
- Title: Bioactive natural products from Okinawa marine organisms
6.1.4. Seminar 4
- Date: December 19, 2014
- Venue: OIST campus
- Speaker: Professor Dr. Hiroshi Tomoda, Graduate School of Pharmaceutical Science, Kitasato University, Japan
- Title: Discovery of useful bioactive compounds from microorganisms
- Supported by: Okinawa Intellectual Cluster Program
6.1.5. Seminar 5
- Date: March 9, 2015
- Venue: OIST campus
- Speaker: Professor Dr. Masayuki Oda, Kyoto Prefectural University, Japan
- Title: Thermodynamic and kinetic analyses for understanding conformational flexibility and function of protein
7. Other
7.1. Outreach Activities
Date: March 19, 2015
Research introduction to students from Nanyang Polytechnic in Singapore and Okinawa National College of Technology
Venue: OIST auditorium
Speaker: Chouthaiwale, P. V.; Tanaka, F.



