FY2019 Annual Report
Micro/Bio/Nanofluidcs Unit
Professor Amy Shen

Abstract
In FY2019, Micro/Bio/Nanofluidics Unit (MBNU) continued to develop two core research themes: fundamental aspects of micro- and nanofluidic flows, coupled with rheology; microfluidics and technologies at the nano-biointerface. Members of the MBNU unit published 18 peer-reviewed papers in FY2019, with our research highlighted in Science Daily, Med Gadget, Health Medicine Network, Science & Technology Research News, etc.
The unit members have also actively disseminated our research results to the general public and scientific communities. We have given 40 presentations and seminars in FY2019, participated core professional society meetings such as the micro-TAS, Annual European Rheology Conference (AERC), Society of Rheology Annual Meeting, and the APS-DFD meeting. Our unit members have also given numerous general science lectures to local schools. Our unit organized an OIST mini-symposium, titled "Fluid-Structure Interactions: From Engineering to Biomimetic Systems” in January 2020. In addition, together with Professor Kenji Takizawa from Waseda University, Professor Shen was a co-organizer of Advances in Computational Fluid–Structure Symposium (AFSI, 2019) in June 2019. We also hosted 16 international scientists who gave research seminars in FY2019, with topics ranging from microfluidics, rheology, applied math, and bio-MEMs.
Personnel wise, Dr. Nikhil Bhalla started an Assistant Professor position in May 2019 at Ulster University in UK. We welcomed 4 new postdocs during the FY2019: Dr. Daniel Carlson from University of Massachusetts, USA; Dr. Vikram Rathee from Georgetown University, USA; Dr. Vincenzo Calabrese from University of Bath, UK; Dr. Charlotte De Blois de la Calande from ESPCI, France. Two Ph.D students (Paul Tsai and Noa Burshtein) defended their Ph.D thesis in January and March of 2020 respectively. Joyce Huang joined the team of our proof-of-concept project in December 2019, to continue the development of "Nanocubes". Our unit also hosted 4 rotation students and 3 research interns from Europe and India.
1. Group Members
As of March 31, 2020
• Prof. Amy Shen, Professor
• Dr. Simon Haward, Group Leader
• Dr. Atsushi Matsumoto, Postdoctoral Scholar
• Dr. Riccardo Funari, Postdoctoral Scholar
• Dr. Cameron Hopkins, Postdoctoral Scholar
• Dr. Daniel Carlson, Postdoctoral Scholar
• Dr. Vikram Rathee, Postdoctoral Scholar
• Dr. Charlotte de Blois, Postdoctoral Scholar
• Dr. Vincenzo Calabrese, Postdoctoral Scholar
• Mr. Kazumi Toda-Peters, Technician
• Ms. Chua Zu Hang, Technician
• Ms. Noa Burshtein, Graduate Student
• Ms. Shivani Sathish, Graduate Student
• Mr. San To Chan, Graduate Student
• Ms. Ainash Garifullina, Graduate Student
• Mr. Kang-Yu Chu, Graduate Student (rotation)
Alumni
• Dr. Hsieh-Fu Tsai, Former Graduate Student (OIST Junior Research Fellow)
• Mr. Ting-Chun Chou, Technician
• Ms. Emily Maria, Rotation Student (Term 1)
• Mr. Mr. Mohamed Abdelgawad, Rotation Student (Term 1)
• Mr. Smith Barnaby, Rotation Student (Term 3)
• Mr. Alexander Groot, Research Intern (Eindhoven University of Technology)
• Mr. Camilo Ijspeert, Research Intern (University of Twente)
• Mr. Rameez Sk Iqbal, Research Intern (Indian Institute of Technology Madras)
2. Collaborations
- Type of collaboration: Joint research
- Researchers:
- Professor Anke Lindner, ESPCI Paris, France
- Professor Norbert Willenbacher, Karlsruhe Institute of Technology, Germany
- Professor Sangwoo Shin, University of Hawaii, USA
- Professor Jesse Ault, Brown University, USA
- Professor Tsutomu Takahashi, Nagaoka University of Technology, Japan
- Professor Aram Chung, Korea University, Korea
- Professor Ashis Sen, IIT Madras, India
- Professor Amy Shen, OIST
- Dr. Simon Haward, OIST
- Dr. Steffen Recktenwald, Karlsruhe Institute of Technology, Germany
3. Activities and Findings
3.1 Vortex dynamics in microfluidics
3.1.1 Coupling of vortex breakdown and stability in a swirling flow
Santo Chan, Jesse T. Ault, Simon J. Haward, Eckart Meiburg, and Amy Q. Shen, "Coupling of vortex breakdown and stability in a swirling flow", Physical Review Fluids, 4, 084701, (2019).
Swirling flows are ubiquitous over a large range of length scales and applications including micron-scale microfluidic devices up to geophysical flows such as tornadoes. As the viscous dissipation, shear, and centrifugal stresses interact, such flows can often exhibit unexpected fluid dynamics. Here, we use microfluidic experiments and numerical simulations to study the flow in a vortex T-mixer: a T-shaped channel with staggered, offset inlets. The vortex T-mixer flow is characterized by a single dominant vortex, the stability of which is closely coupled to the appearance of vortex breakdown. Specifically, at a Reynolds number of Re ≈ 90, a first vortex breakdown region appears in the steady-state solution, rendering the vortex pulsatively unstable. A second vortex breakdown region appears at Re ≈ 120, which restabilizes the vortex. Finally, a third vortex breakdown region appears at Re ≈ 180, which renders the vortex helically unstable. Thus, a counterintuitive flow regime exists for the vortex T-mixer in which increasing the Reynolds number has a stabilizing effect on the steady-state flow. The pulsatively unstable vortex evolves into a periodically pulsating state with a Strouhal number of St ≈ 0.5, and the helically unstable vortex evolves into a helically oscillating state with St ≈ 1.75. These transitions can be explained within the framework of linear hydrodynamic stability. This study provides experimental and numerical evidence of the close coupling between vortex breakdown and flow stability, including the restabilization of the flow with increasing Reynolds number due to the appearance of a vortex breakdown region, which will provide new insights into how vortex breakdown can affect the stability of a swirling flow.
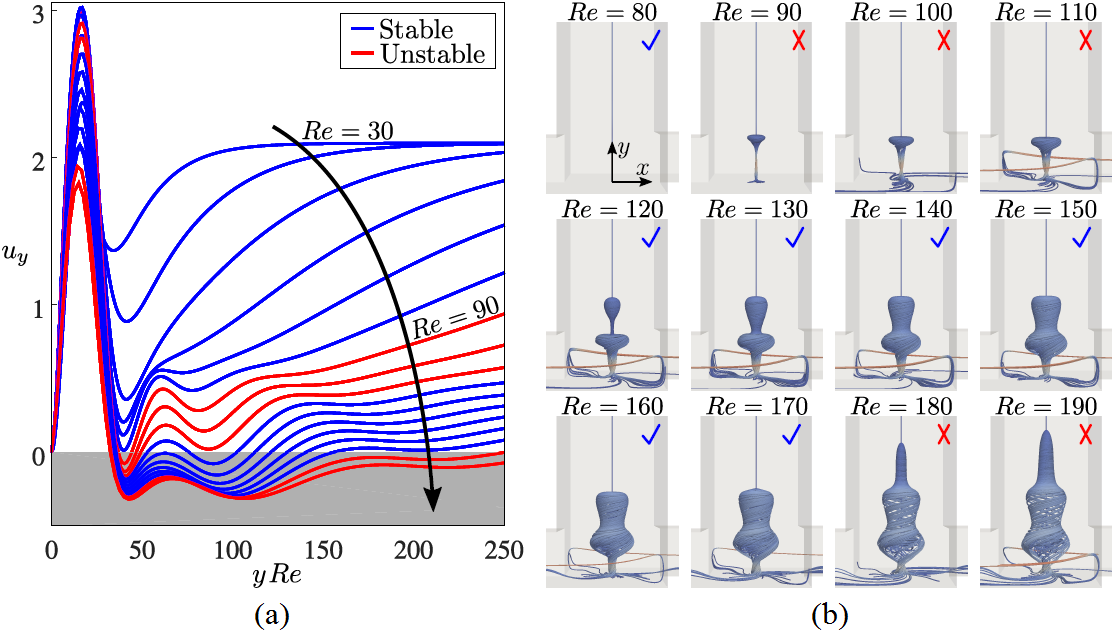
Figure 1: Stability of the steady-state solutions. (a) Distributions of dimensionless axial velocity uy of the steady-state solutions along the channel centerline. (b) 3D streamline visualizations of the vortex breakdown regions in the outlet channel as a function of Re. Blue check marks indicate stable solutions and red crosses indicate unstable solutions.
3.1.2 Particle trapping in merging flow junctions by fluid-solute-colloid-boundary interactions
Sangwoo Shin, Jesse T. Ault, Kazumi Toda-Peters, and Amy Q. Shen, "Particle trapping in merging flow junctions by fluid-solute-colloid-boundary interactions ", Physical Review Fluids, 4, 084701, (2019).
Merging of different streams in channel junctions represents a common mixing process that occurs in systems ranging from soda fountains and bathtub faucets to chemical plants and microfluidic devices. Here, we report a spontaneous trapping of colloidal particles in a merging flow junction when the merging streams have a salinity contrast. We show that the particle trapping is a consequence of nonequilibrium interactions between the particles, solutes, channel, and the freestream flow. A delicate balance of transport processes results in a stable near-wall vortex that traps the particles. We use three-dimensional particle visualization and numerical simulations to provide a rigorous understanding of the observed phenomenon. Such a trapping mechanism is unique from the well-known inertial trapping enabled by vortex breakdown, or the solute-mediated trapping enabled by diffusiophoresis, as the current trapping is facilitated by both the solute and the inertial effects, suggesting a new mechanism for particle trapping in flow networks.
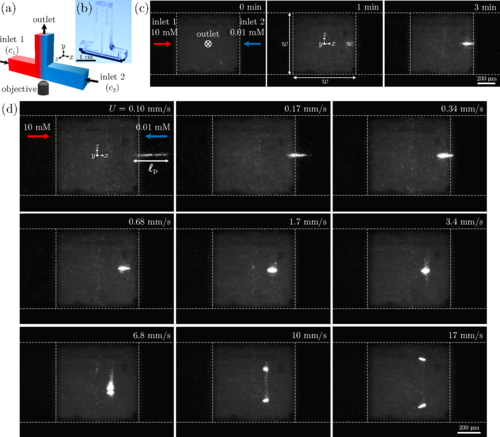
Figure 2. Particle trapping induced by merging of different saline solutions at a T-junction. (a) Schematic of a symmetric T- junction with merging flows of solute concentrations c1 and c2. (b) A photograph of a glass microfluidic T-junction device made by selective laser-induced etching. (c) Image sequence of fluorescent colloidal particles (carboxylate-modified polystyrene latex, 1 μm in diameter) accumulating near the junction by merging of two colloidal suspensions with different solute concentrations (NaCl, c1 = 10 mM, c2 = 0.01 mM). Inlet mean flow speed is U = 0.34 mm/s. The channel has a square cross section with width w = 0.7 mm throughout the entire channel. (d) Particle trapping versus the inlet mean flow speed.
3.1.3. Intracellular Nanomaterial Delivery via Spiral Hydroporation
GeoumYoung Kang, Daniel W. Carlson, Tae Ho Kang, Seungki Lee, Simon J. Haward, Inhee Choi, Amy Q. Shen, and Aram J. Chung, "Intracellular Nanomaterial Delivery via Spiral Hydroporation", ACS Nano, 14, 3, 3048, (2020).
This work reports on the development of an intracellular nanomaterial delivery platform, based on unexpected cell-deformation phenomena via spiral vortex and vortex breakdown exerted in the cross- and T-junctions at moderate Reynolds numbers. These vortex-induced cell deformation and sequential restoration processes open cell membranes transiently, allowing effective and robust intracellular delivery of nanomaterials in a single step without the aid of carriers or external apparatus. By using the platform described here (termed spiral hydroporator), we demonstrate the delivery of different nanomaterials, including gold nanoparticles (200 nm diameter), functional mesoporous silica nanoparticles (150 nm diameter), dextran (hydrodynamic diameters between 2–55 nm), and mRNA, into different cell types. We demonstrate that the system is highly efficient (up to 96.5%) with high throughput (up to 1 × 106 cells/min) and rapid delivery (∼1 min) while maintaining high levels of cell viability (up to 94%).
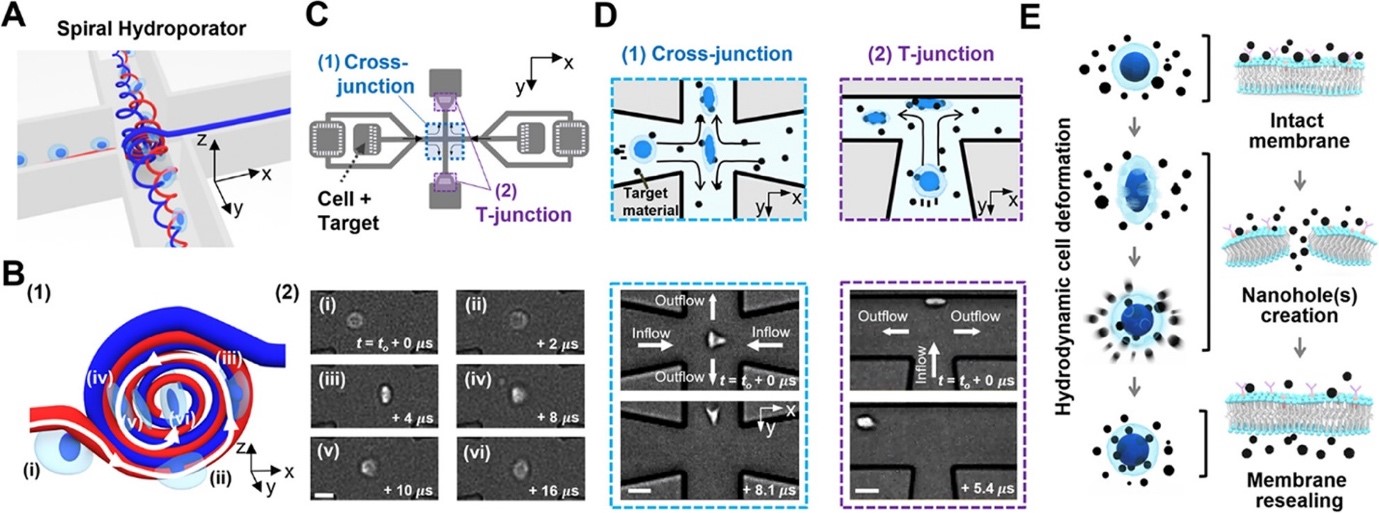
Figure 3: Spiral hydroporation for intracellular nanomaterial delivery. (A) Schematic depicting spiral flow motion at a cross-junction channel. (B) (1) Illustration of spiral vortex-induced cell deformation and (2) high-speed microscope images showing rotational cell motions (scale bar: 10 μm). (C) CAD layout of the hydroporation microfluidic device, consisting of (1) a cross-junction and (2) two dividing T-junction channels. (D) Hydrodynamic cell deformation via (1) the spiral vortex at the cross-junction and (2) cell-wall collision at the T-junction(s) (scale bars: 20 and 30 μm for (1) and (2), respectively). (E) Schematic illustrating nanomaterial delivery into cell cytosols.
3.2 Bifurcation and instability in microscopic flows of complex fluids
3.2.1 Purely elastic fluid-structure interactions in microfluidics
Cameron C. Hopkins, Simon J. Haward, Amy Q. Shen, "Purely elastic fluid-structure interactions in microfluidics: implications for mucociliary flows", Small, 3, 1903872 (2019).
Fluid-structure interactions lie at the heart of the complex, and often highly coordinated, motions of actively driven micro-scale biological systems (e.g. translating cilia, flagella and motile cells). Due to the highly viscoelastic nature of most relevant biological fluids and the small length scales involved, the viscous and inertial forces in such flows are dominated by elasticity. However, elastic effects are often overlooked in studies seeking to address phenomena like the synchronization of beating cilia. In this study, unique microfluidic experiments are presented to demonstrate that inertia-free viscoelastic flows can lead to highly regular beating of an immersed (passive) flexible structure, herein named “purely-elastic” fluid-structure interaction. It is also shown how two such flexible structures can achieve an extraordinary degree of synchronization, with a correlation coefficient approaching unity. The synchronization is a result of the generation of localized elastic stresses in the fluid that effectively link the two objects. These purely elastic interactions may be important to consider toward developing a complete understanding of the motions of microscale biological systems.
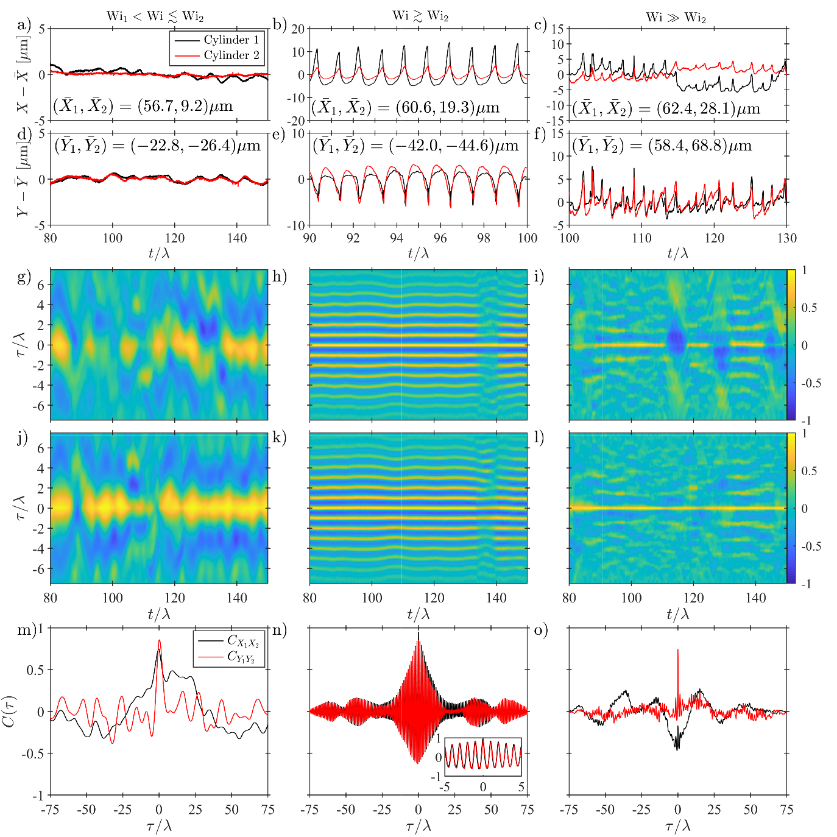
Figure 4: Analysis of time-dependent motions of the two cylinders measured in the Double-Cylinder (DC) flow channel at three representative values of the Weissenberg number. (a) – (c) time series of the X positions of the cylinders. (d) – (f) time series of the Y positions of the cylinders. (g) – (i) time-resolved correlation functions between the X positions of DC1 and DC2. (h) – (l) time-resolved correlation functions between the Y positions of DC1 and DC2. (m) – (n) full, post transient, experimental time duration correlation functions between the X and Y positions of DC1 and DC2.
3.2.2 Microfluidic Analog of an Opposed-Jets Device
Simon J. Haward, Cameron C. Hopkins, Kazumi Toda-Peters, Amy Q. Shen, "Microfluidic Analog of an Opposed-Jets Device", Applied Physics Letters 114, 223701 (2019).
A fully three-dimensional (3D) stagnation point microfluidic device is fabricated that, similar to the classical opposed-jet apparatus, can be operated in either a uniaxial or a biaxial extensional flow mode with an easily controllable strain rate. The microchannel is etched inside fused silica and has optical access through all three planes. A detailed characterization of the Newtonian flow field by microparticle image velocimetry confirms the expected nature of the flow and compares well with the prediction of 3D numerical simulations. Flow-induced birefringence of a model polymer solution demonstrates the extension of macromolecules in both modes of operation and the potential use of the device for quantitative rheo-optical studies. This microfluidic opposed jet device could also be used for examining the deformation and dynamics of drops, cells, fibers, and single molecules in well-defined and relevant flow fields.
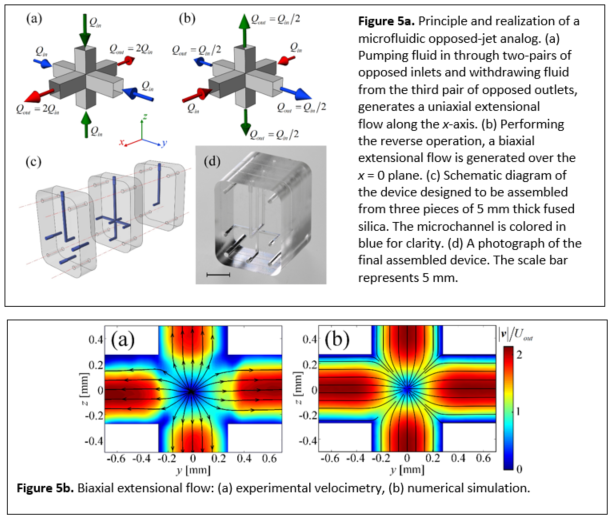
3.2.3 Asymmetric flow of polymer solutions around microfluidic cylinders: Interaction between shear-thinning and viscoelasticity
Simon J. Haward, Cameron C. Hopkins, Amy Q. Shen, "Asymmetric flow of polymer solutions around microfluidic cylinders: Interaction between shear-thinning and viscoelasticity ", Journal of Non-Newtonian Fluid Mechanics, 278, 104250, (2020).
Viscoelastic flow around a cylinder is a model problem representing a wide range of relevant industrial processing and biological applications. Reducing the cylinder to microscopic dimensions conveniently enables the problem to be examined in the absence of inertia. Recently, we have developed glass microfluidic geometries containing long and slender, yet rigidly fixed, microfluidic cylinders, which present a low blockage ratio β = 2r/W = 0.1, where r = 20 μm is the cylinder radius and W = 400 μm is the channel width. Using a shear-banding viscoelastic wormlike micellar (WLM) solution, we showed how the flow around such a cylinder can destabilize beyond a critical Weissenberg number (Wi = λU/r, where λ is a characteristic time of the fluid and U is the average flow velocity), resulting in the asymmetric division of the fluid around either side of the cylinder [Haward et al, Soft Matter 15:1927]. In the present work we investigate this flow instability in greater detail using a range of polymer solutions formulated from hydrolyzed poly(acrylamide) (HPAA) dissolved over a range of concentration in deionized water. The test fluids present a range of shear-thinning responses under steady shear, and also a wide range of characteristic times. At low HPAA concentrations, the flow around the cylinder remains essentially symmetric for all Wi, but as the concentration increases, so does the maximum degree of the flow asymmetry observed with increasing Wi. Interestingly, at intermediate concentrations, the flow can resymmetrize at very high Wi. We understand these effects by considering simultaneously both the degree of shear-thinning of the fluid and the imposed Wi, and our analysis shows that both strong shear-thinning and high elasticity are required for the formation of strongly asymmetric flows. Our results represent the first report of this highly asymmetric flow state in polymer solutions, showing that it is a general phenomenon and not only specific to WLM. Our analysis provides a clear insight into the origins of this unusual flow state and may also be relevant to understanding other instances of asymmetries arising in shear-thinning viscoelastic flows.

3.3 Developing Biosensors at the nano-bio-interface
Our unit has been developing various sensor platforms (i.e., plasmonic, QCM) whereas nanofabrication, surface chemistry, and device integration are equally important to achieve superior biosensing performance.
3.3.1 Optimized Immobilization of Biomolecules on Gold Nanostructures for Biosensing
Ainash Garifullina and Amy Q. Shen, "Optimized Immobilization of Biomolecules on Nonspherical Gold Nanostructures for Efficient Localized Surface Plasmon Resonance Biosensing", Analytical Chemistry, 91(23): 15090-15098 (2019).
Plasmonic biosensing techniques employ metal nanostructures, commonly gold (Au), often with biomolecules attached to their surfaces either directly or via other linkers. Various surface chemistry methods based on dispersion and covalent interactions are used to attach biomolecules to Au. Thus, when immobilizing a molecule on a metal surface, quantitative estimates of binding efficiency and stability of these surface chemistry methods are needed. Most prior work to compare such methods deals with bulk/thin film configurations or spherical nanoparticles, and very little is known about immobilization of biomolecules on plasmonic nanostructures of different shapes. In our work, we compared four representative Au surface functionalization methods as well as estimated how efficient these methods are at attaching biomolecules to nonspherical plasmonic Au nanostructures. We estimated the concentration of immobilized antibody that is specific to human C-reactive protein (anti-hCRP) by measuring the localized surface plasmon resonance (LSPR) shifts after exposing the surface of Au nanostructures to the antibody (Fig. 7).
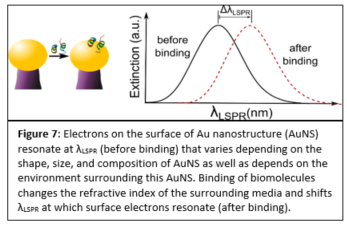
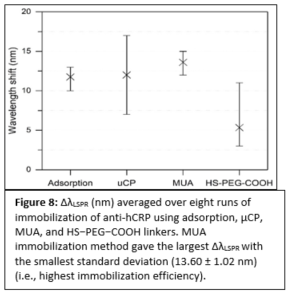
Our results differ from the previously reported ones since the highest concentration of anti-hCRP was immobilized using 11-mercaptoundecanoic acid (MUA) chemistry (Fig. 8). We demonstrated that immobilized antibodies retained their stability and specificity toward hCRP throughout the immunoassay when diluted hCRP or hCRP-spiked human serum samples were used. These findings have important implications for the fields of biosensing and diagnostics that employ nonspherical plasmonic nanostructures since an overall performance of these devices depends on efficient biomolecule immobilization.
3.3.2 High-Throughput Protein Immobilization for Miniaturized Bioassays
Shivani Sathish, Noriko Ishizu, and Amy Q. Shen, "Air Plasma-Enhanced Covalent Functionalization of Poly(methyl methacrylate): High-Throughput Protein Immobilization for Miniaturized Bioassays", ACS Applied Materials and Interfaces, 11(49): 46350-46360, (2019).
Poly(methyl methacrylate) (PMMA) is being increasingly used for the fabrication of biomicrofluidic platforms. However, the low surface energy of PMMA triggers biomolecule physisorption via hydrophobic interactions that not only increases non-specific adsorption but also enhances biomolecule denaturation. Acid/base treatments are commonly used to generate functional groups (e.g., amines or carboxyls) that enable covalent coupling of biomolecules on PMMA. These surface treatments have been shown to change both the topography and transparency of the PMMA substrates. On the other hand, low-pressure radio frequency (RF) air plasma has been employed for surface hydrophilization of polymers, including PMMA, to increase surface wettability for various applications. Here, we selectively exploited the carboxyls generated on PMMA surfaces when exposed to RF air plasma, for covalent coupling of proteins via simple 1-ethyl-3-(3-(dimethylamino)-propyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) chemistry. Owing to its intrinsic ability to exhibit changes in fluorescence emission when exposed to protein denaturating conditions, we used green fluorescence protein (GFP) as a model following immobilization. We observed that the surface density of functional GFP on PMMA can be tailored by protein system to quantify the preservation of biomolecular structure and biofunctionality altering the plasma energy (E), initial GFP concentration (C0) and pH of the protein buffer. Finally, we biofunctionalized PMMA microfluidic antibody assay devices and successfully detected clinically significant concentrations of Chlamydia trachomatis specific antibodies.
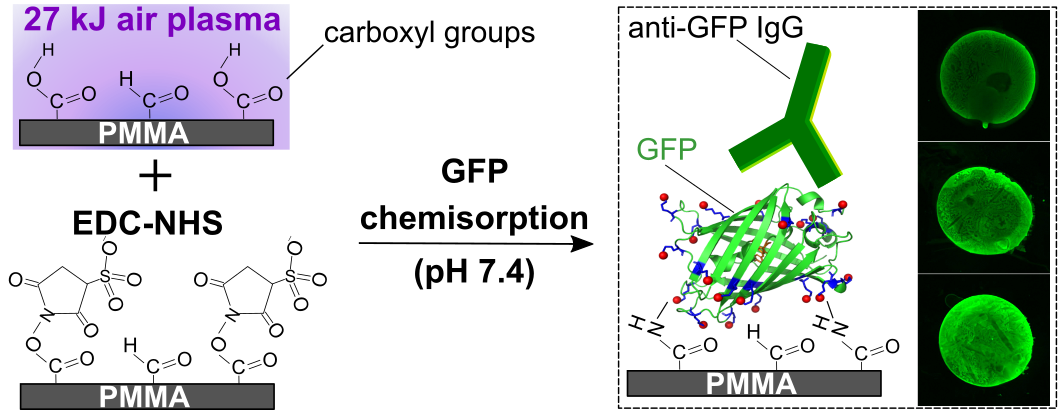
Figure 9: Schematic illustrating the conditions that allow covalent immobilization of GFP on air plasma-activated PMMA. Fluorescent images depict the binding of anti-GFP immunoglobulins to GFP molecules immobilized on treated PMMA surfaces.
3.3.3 Effect of Substrate on Refractive Index Sensitivity of Nanoplasmonic Gold
Nikhil Bhalla, Aditya Jain, Yoonjoo Lee, Amy Q. Shen and Doojin Lee, "Dewetting Metal Nanofilms—Effect of Substrate on Refractive Index Sensitivity of Nanoplasmonic Gold", Nanomaterials, 9(11): 1530, (2019).
This work describes some of the fundamental properties of materials which influence the refractive index sensing properties of the nanoplasmonic materials. Usually nanostructures of noble metals such as gold and silver, that show Localized Surface Plasmon Resonance (LSPR), are fabricated on a supporting substrate such as glass or silicon based materials with different shapes, sizes and spacing. This work revealed that the silicon-based supporting substrate influence the LSPR associated refractive index sensitivity of gold (Au) nanostructures designed for sensing applications. For our case study, among the silicon based substrates, Au nanostructures were fabricated on four different silicon-based ceramic substrates (Si, SiO2, Si3N4, SiC) by thermal dewetting process. We use a simple thermal dewetting procedures as it offers cost effective and time efficient solutions for fabrication of the plasmonic nanostructures on a given substrate. Among these Si-supported Au plasmonic refractive index (RI) sensors, the Au nanostructures on the SiC substrates display the highest average RI sensitivity of 247.80 nm/RIU, for hemispherical Au nanostructures of similar shapes and sizes. This is 4 times higher than the sensitivities of Au nanostructures which typically depict sensitivity of less than 60 nm/RIU. The results were further validated by finite element analysis using simulation tools for plasmonics.

Figure 10: Gold nanoparticle and its electric field distribution due to localized plasmons. The bottom substrate shows how its guided mode fields interact with plasmons on gold, which eventually leads to LSPR sensitivity enhancement for refractive index sensing applications.
3.3.4 Real-time monitoring of DNA immobilization & detection of DNA polymerase activity
Johanna Roether, Kang-Yu Chu, Norbert Willenbacher, Amy Q. Shen and Nikhil Bhalla, "Real-time monitoring of DNA immobilization and detection of DNA polymerase activity by a microfluidic nanoplasmonic platform", Biosensors and Bioelectronics, 142: 111528, (2019).
This work investigates the integration of microfluidics with nanoplasmonics for the use in DNA biosensing. The Localized Surface Plasmon Resonance (LSPR) was integrated with microfluidics to enhance both the sensitivity of the LSPR transduction process and the efficiency of polymerase enzyme activity. In addition, this platform can precisely control the minute volumes involved in various steps on polymerase activity to facilitate translation of LSPR into portable microfluidic chip formats for point-of-care biosensing applications. Although the development microfluidic chip was used for DNA reactions, the chip is also suitable to meet the demand of real-time monitoring for a wide range of bioassays ranging from protein-protein interactions, antibody-antigen kinetics or for screening candidate compounds for drug discovery. Furthermore, the chip eliminates some loopholes of the current labeled sensing approaches developed to detect DNA polymerase which includes discontinuous radio-labeled, direct and indirect fluorescence and particle labeled assays at bulk and single molecule level. As most of these methods are either time consuming, laborious, cost inefficient or require the usage of toxic chemical reagents (e.g., radioactive tags/ labels) the chip developed in this work represent an important innovation in the field of micro/nanotechnologies.
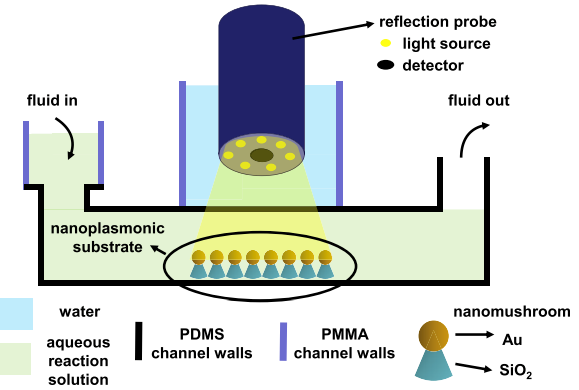
Figure 11: The schematic shows how the reflection probe is integrated with the microfluidic chip consisting of gold nanomushrooms which act as LSPR sensors for the detection of analyte under test. The stacked layers of the PDMS and PMMA are used for the device integration.
3.3.5 Real-time Detection of Gold Biomineralization
Riccardo Funari, Rosa Ripa, Bill Söderström, Ulf Skoglund and Amy Q. Shen, "Detecting Gold Biomineralization by Delftia acidovorans Biofilms on a Quartz Crystal Microbalance", ACS Sensors, 4: 3023-3033, (2019).
The extensive use of gold in sensing, diagnostics, and electronics has led to major concerns in solid waste management since gold and other heavy metals are non-biodegradable and can easily accumulate in the environment. Here, by using complementary measurement techniques such as quartz crystal microbalance (QCM), atomic force microscopy, crystal violet staining, and optical microscopy, we investigate the gold biomineralization process accomplished by biofilms of bacterium Delftia acidovorans. When stressed byAu3+ ions, D. acidovorans is able to neutralize toxic soluble gold by excreting a non ribosomal peptide, which forms extracellular gold nanonuggets via complexation with metal ions. Specifically, QCM, a surface-sensitive transducer, is employed to quantify the production of these gold complexes directly on the D. acidovorans biofilm in real time. Detailed kinetics obtained by QCM captures the condition for maximized biomineralization yield and offers new insights underlying the biomineralization process. This is the first study providing an extensive characterization of the gold biomineralization process by a model bacterial biofilm. We also demonstrate QCM as a cheap, user-friendly sensing platform and alternative to standard analytical techniques for studies requiring high-resolution quantitative details, which offers promising opportunities in heavy-metal sensing, gold recovery, and industrial waste treatment.
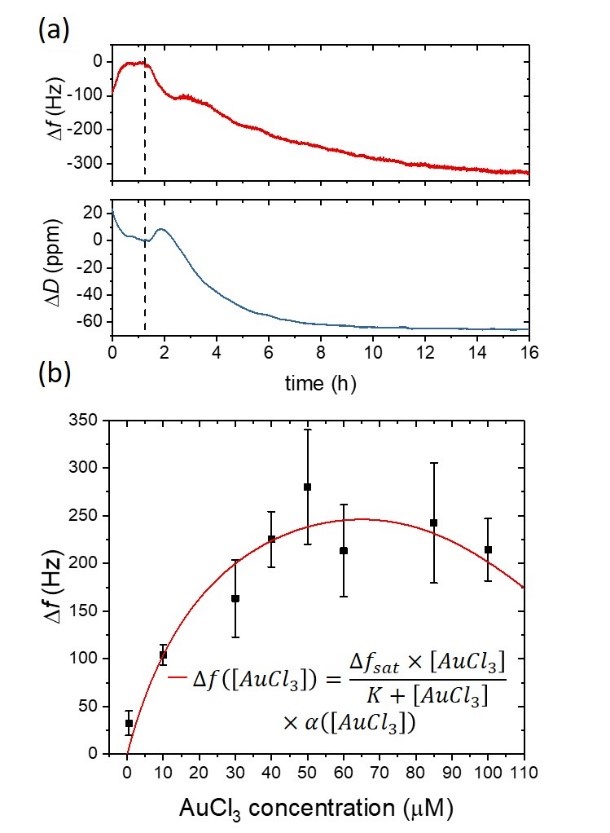
Figure 12: D. acidovorans response at several concentrations of AuCl3 monitored using QCM. (a) The microorganisms are then stressed with soluble gold (50 μM AuCl3), and both frequency and dissipation are monitored for more than 14h. The gold biomineralization and the subsequent gold nanonuggets production yield a progressive decrease in the resonant frequency of the gold coated quartz substrate due to the deposition of gold complexes on the QCM sensor. A decrease in the dissipation signal is also observed, which indicates a gradual stiffening of the material that is in contact with the QCM sensor surface. (b) Frequency response of the biofilm-covered QCM sensor surface at different concentrations of the soluble gold.
3.3.6 Detecting Escherichia coli Biofilm Development Stages on Gold and Titanium
Rosa Ripa, Amy Q. Shen and Riccardo Funari, "Detecting Escherichia coli Biofilm Development Stages on Gold and Titanium by Quartz Crystal Microbalance", ACS Omega, 5, 2295-2302, (2020).
Bacterial biofilms are responsible for persistent infections and biofouling, raising serious concerns in both medical and industrial processes. These motivations underpin the need to develop methodologies to study the complex biological structures of biofilms and prevent their formation on medical implants, tools, and industrial apparatuses. Here, we report the detailed comparison of Escherichia coli biofilm development stages (adhesion, maturation, and dispersion) on gold and titanium surfaces by monitoring the changes in both frequency and dissipation of a quartz crystal microbalance (QCM) device. The QCM outcomes are further confirmed by atomic force microscopy and crystal violet staining, thus validating the effectiveness of this surface sensitive sensor for microbial biofilm research. Moreover, because QCM technology can easily modify the metal types and coatings, QCM sensors also provide well-controlled experimental conditions to study antimicrobial surface treatments and eradication procedures, even on mature biofilms.
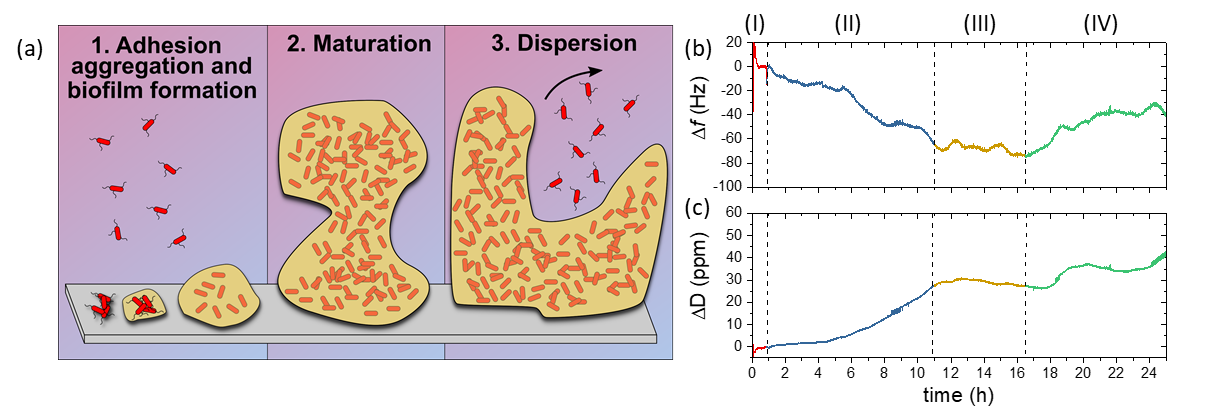
Figure 13: (a) Stages of biofilm development. Bacteria appendages drive cell adhesion onto solid surfaces (1). This interaction is stabilized by the production of extracellular polymeric substances (EPSs), which improve the attachment and offer both mechanical and chemical protection for the bacteria. Biofilm growth and maturation (2) is followed by the release of free-floating bacteria for further colonization (3). (b) Frequency and (c) Dissipation QCM sensorgrams of E. coli growing on gold. The color scheme highlights the stages of the experiment: initial stabilization with culture medium [red, (I)], biofilm formation [blue, (II)], maturation [yellow, (III)], and release of free planktonic cells [green, (IV)].
3.4 Microfluidics for biomedical engineering applications
3.4.1 Modular Fluid Handling Prototype Integrated with Microfluidic Biochemical Assay Modules for Point-of-Care Testing
Shivani Sathish, Kazumi Toda-Peters, and Amy Q. Shen, "Proof-of-Concept Modular Fluid Handling Prototype Integrated with Microfluidic Biochemical Assay Modules for Point-of-Care Testing”, VIEW, DOI: 10.1002/viw2.1 (2020).
Most common at-home infectious disease tests are designed to force users to independently collect fluid samples, manually measure and mix specified reagents, and perform assays using the allocated components of the kits. Although these kits offer the advantage of obtaining the assessments within a few minutes to a few hours, the likelihood of obtaining false positives and erroneous analyses is high. To overcome these issues, we are developing a modular system consisting of two components: (i) the Fluid Handling Device module (FHD) and (ii) an interchangeable assay module. The FHD is designed to allow users to collect and mix a fluid sample with pre-loaded reagents, filter the mixture, sequentially deliver the processed sample to an on-board microfluidic assay device and collect the fluid wastes, within a sealed system, thereby reducing user-induced variability and contamination, while obtaining the assay results within a few minutes. After the completion of the tests, the user can simply dispose the sealed prototype as designated laboratory waste.
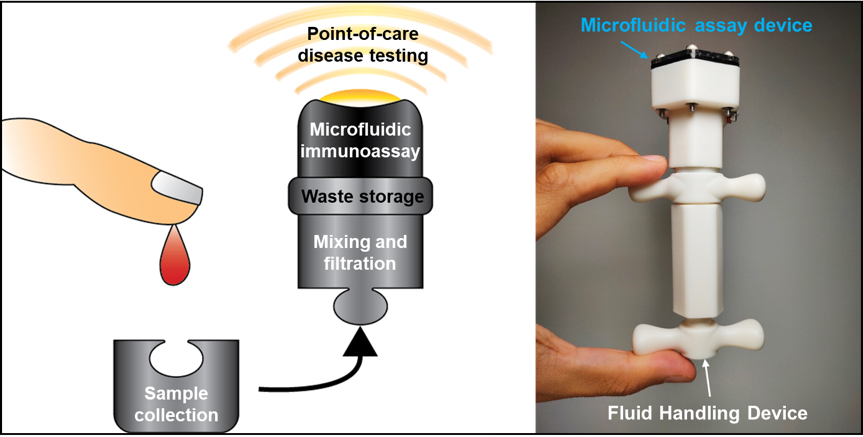
Figure 14: The palm-sized device is an integrated, completely sealed and disposable point-of-care testing prototype that exploits the benefits of microfluidics and 3D-printing fabrication techniques. It consists of a manually-operated Fluid Handling Device that allows precise mixing, filtration and delivery of fluids to an on-board microfluidic assay unit for subsequent detection of specific biochemical analytes, with minimal risks of contamination.
3.4.2 Voltage-gated ion channels mediate the electrotaxis of glioblastoma
H.-F. Tsai, Camilo IJspeert, Amy Q. Shen, "Voltage-gated ion channels mediate the electrotaxis of glioblastoma in a hybrid PMMA/PDMS microdevice”, submitted, (2020). BioRxiv, 2020.02.14.948638.
Transformed astrocytes in the most aggressive form cause glioblastoma, the most common cancer in central nervous system with high mortality. The physiological electric field by neuronal local field potentials and tissue polarity may guide the infiltration of glioblastoma cells through the electrotaxis process. However, microenvironments with multiplex gradients are difficult to create. In this work, we have developed a hybrid microfluidic platform to study glioblastoma electrotaxis in controlled microenvironments with high throughput quantitative analysis by a machine learning-powered single cell tracking software. The electrotaxis of two glioblastoma models, T98G and U-251MG, require optimal laminin-containing extracellular matrix and exhibits opposite directional and electro-alignment tendencies. Calcium signaling is a key contributor in glioblastoma pathophysiology but its role in glioblastoma electrotaxis is still an open question. Anodal T98G electrotaxis and cathodal U-251MG electrotaxis require the presence of extracellular calcium cations. U-251MG electrotaxis is dependent on the P/Q-type voltage-gated calcium channel (VGCC) and T98G is dependent on the R-type VGCC. U-251MG and T98G electrotaxis are also mediated by A-type (rapidly inactivating) voltage-gated potassium channels and acid-sensing sodium channels. The involvement of multiple ion channels suggests that the glioblastoma electrotaxis is complex and patient-specific ion channel expression can be critical to develop personalized therapeutics to fight against cancer metastasis.
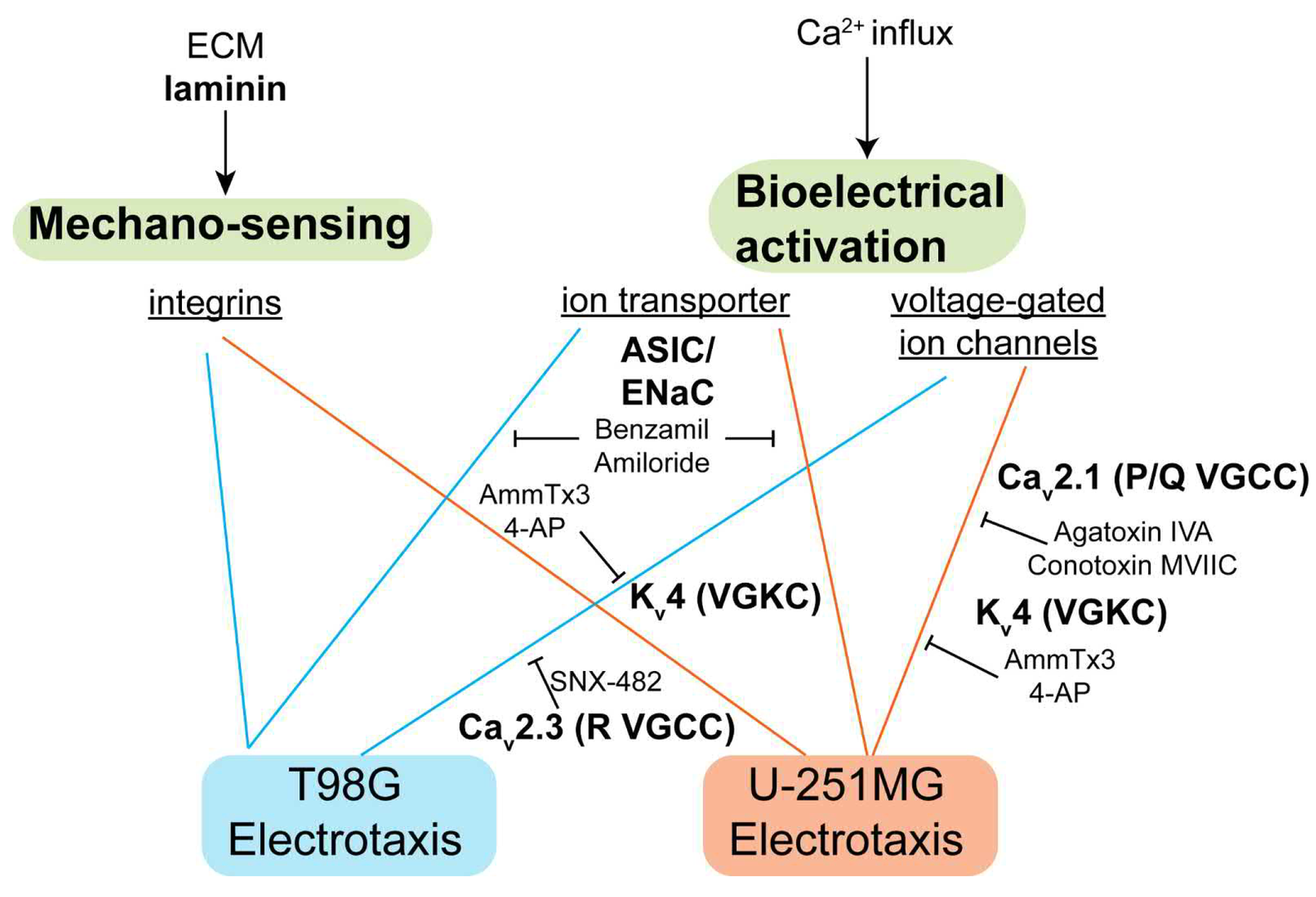
Figure 15: The signaling mechanism identified in this study. Laminin-based ECMs is necessary for glioblastoma electrotaxis, suggesting that integrins may play a role. The voltage-gated ion channels and ion transporters also mediate glioblastoma electrotaxis that requires extracellular calcium.
3.5 General topics in Interfacial Science and Rheology
3.5.1. Substrate stiffness on particle drying patterns
Rameez Iqbal, Atsushi Matsumoto, A. Sudeepthi, Amy Q. Shen and Ashis K. Sen, "Substrate stiffness affects particle distribution pattern in a drying suspension droplet”, Applied Physics Letters, 114, 253701,(2019).
Droplet evaporation is ubiquitous and has led to a plethora of applications owing to the formation of several distinct deposition patterns. We report the role of substrate elastic modulus on the final morphological pattern of an evaporating suspension droplet. The final deposition pattern of the droplet was found to increase with the reduction in the substrate elastic modulus. The particles on the stiffer substrate are deposited in a closed pack multilayered pattern whereas for the softer substrate, a loosely packed, uniform monolayer deposition with a finite gap between the adjacent particles are observed. This transition from the centralized (multilayer) to the uniform (monolayer) deposition pattern could be attributed to the prolonged pinning owing to the higher potential energy barrier offered by the substrate due to the surface asperities, chemical inhomogeneities and most importantly to the wetting ridge (see Figure 16). The outcome of the present study may find applications in biochemical characterization and analysis of micro/nano particles.
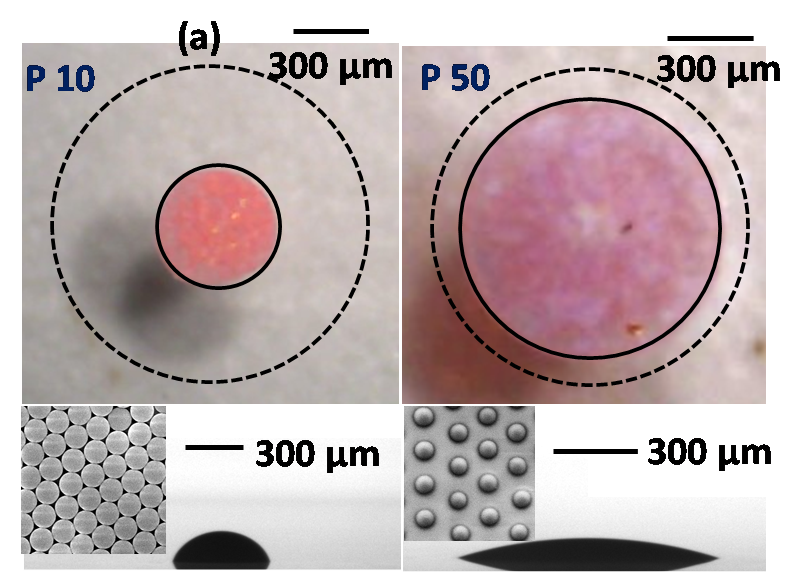
Figure 16: Evaporative deposition pattern of a colloidal suspension droplet on a rigid (P10) and soft (P50) substrate. The dotted line in the top view indicates the initial contact diameter of the droplet while dispensing, whereas the bottom figure indicates the side view of the final deposition pattern.
4. Publications
4.1 Journals
- GeoumYoung Kang, Daniel W. Carlson, Tae Ho Kang, Seungki Lee, Simon J. Haward, Inhee Choi, Amy Q. Shen, Aram J. Chung, Intracellular nanomaterial delivery via spiral hydroporation, ACS Nano, 14, 3048-3058, (2020).
- Sangwoo Shin, Jesse T. Ault, Kazumi Toda-Peters, and Amy Q. Shen, Particle trapping in merging flow junctions by fluid--solute--colloid--boundary interactions, Physical Review Fluids, 5, 024304, (2020).
- Simon J. Haward, Cameron C. Hopkins, Amy Q. Shen, Asymmetric flow of polymer solutions around microfluidic cylinders: Interaction between shear-thinning and viscoelasticity, Journal of Non-Newtonian Fluid Mechanics, 278, 104250, (2020).
- Rosa Ripa, Amy Q. Shen, and Riccardo Funari, Detecting E. coli biofilm development stages on gold and titanium by a quartz crystal microbalance, ACS Omega, 5, 2295--2302, (2020).
- Shivani Sathish, Kazumi Toda-Peters, and Amy Q. Shen, Proof-of-Concept modular fluid handling prototype integrated with microfluidic biochemical assay modules for point-of-care testing, VIEW, 1, e1, (2020).
- Cameron Hopkins, Simon J. Haward, and Amy Q. Shen, Purely elastic fluid-structure interactions in microfluidics: implications for mucociliary flows, Small, 1903872, (2019),
- San To Chan, Jesse T. Ault, Simon J. Haward, E. Meiburg, and Amy Q. Shen, Coupling of vortex breakdown and stability in a swirling flow, Physical Review Fluids, 4, 084701, (2019).
- Simon J. Haward, Cameron C. Hopkins, Kazumi Toda-Peters, and Amy Q. Shen, Microfluidic analog of an opposed-jets device, Applied Physics Letters, 114, 223701, (2019).
- R. Iqbal, Atsushi Matsumoto, A. Sudeepthi, Amy Q. Shen, and A. K. Sen, Substrate stiffness affects particle distribution pattern in a drying suspension droplet, Applied Physics Letters, 114, 25370, (2019).
- Noa Burshtein, San To Chan, Kazumi Toda-Peters, Amy Q. Shen, Simon J. Haward, 3D-printed glass microfluidics for fluid dynamics and rheology, Current Opinion in Colloid & Interface Science, 43: 1-14, (2019).
- Shivani Sathish, Noriko Ishizu, and Amy Q. Shen, Air plasma-enhanced covalent functionalization of poly(methyl methacrylate): high-throughput protein immobilization for miniaturized bioassays, ACS Applied Materials & Interfaces, 11, 49, 46350-46360, (2019).
- Ainash Garifullina and Amy Q. Shen, Optimized immobilization of biomolecules on non-spherical Au nanostructures for efficient LSPR biosensing, Analytical Chemistry, (2019).
- Riccardo Funari, Rosa Ripa, Bill Bill Soderstrom, Ulf Skoglund, Amy Q Shen, Detecting gold biomineralization by Delftia acidovorans biofilms on a quartz crystal microbalance, ACS Sensors, 4, 11, 3023-3033, (2019).
- Nikhil Bhalla, Aditya Jain, Yoonjoo Lee, Amy Q. Shen, Doojin Lee, Dewetting Metal Nanofilms—Effect of Substrate on Refractive Index Sensitivity of Nanoplasmonic Gold, Nanomaterials, 9, 1530, (2019).
- Johanna Roether, Kang-Yu Chu, Norbert Willenbacher, Amy Q. Shen, and Nikhil Bhalla, Real-time monitoring of DNA immobilization and detection of DNA polymerase activity by a microfluidic nanoplasmonic platform, Biosensors and Bioelectronics, 142, 111528, (2019).
- Preeya, Kuray, Takeru Noda, Atsushi Matsumoto, Ciprian Iacob, Tadashi Inoue, Michael A. Hickner, and James Runt, Ion Transport in Pendant and Backbone Polymerized Ionic Liquids, Macromolecules, 52, 17, 6438-6448, (2019).
- Konstantinos Zografos, Simon J. Haward, Monica S. N. Oliveira, Optimised multi-stream designs for controlled extensional deformation, Microfluidics and Nanofluidics, 23, 131, (2019).
- Steffen M. Recktenwald, Simon J. Haward, Amy Q. Shen, N Willenbacher, Heterogeneous flow inside threads of low viscosity fluids leads to anomalous long filament lifetimes, Scientific Reports, 9, 7110, (2019).
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Atsushi Matsumoto, Riccardo Funari, John R. de Bruyn, and Amy Q. Shen, Viscoelastic Measurement of Electric Double Layer Using Quartz Crystal Microbalance, The 100th CSJ Annual Meeting, Chiba, Japan, March 2020.
- Daniel W. Carlson, Yahya Modarres-Sadeghi. Flow-induced vibrations for a square prism with crossflow-inline freedom, FSI Mini-symposium, OIST, Okinawa, Japan, January 2020.
- Haward, Simon J., The Asymmetric Flow of Polymer Solutions around Cylinders. OIST mini-symposium “Fluid-Structure Interactions: From Engineering to Biomimetic Systems” OIST, Okinawa, Japan, January 2020.
- Sathish, Shivani, and Shen, Amy Q. Shen, Development of cheap point-of-care devices for early and rapid disease diagnosis, Career Link Meetup, Hokkaido, Japan, December 2019.
- Amy Q. Shen, Microfluidic assisted particle synthesis for drug delivery and imaging applications, The 2019 Asian Chemical Congress, Biomaterials Symposium (RSC), Taipei, December 2019.
- Amy Q. Shen, Using microfluidics to probe complex flows in biomimetic systems, Max Planck Croucher Symposium, Okinawa, November 2019.
- Amy Q. Shen, New observations of transport phenomena around junctions in microfluidic flows, Okinawa Colloids 2019, Okinawa, November 2019.
- Amy Q. Shen, New opportunities to probe complex flows and nanoconfinement achieved by new fabrication techniques, The Bergveld Lecture, University of Twente, The Netherlands, September 2019.
- Amy Q. Shen, New opportunities to probe flow instabilities of complex fluids with novel glass microfluidic devices, Eindhoven University of Technology, The Netherlands, September, 2019.
- Amy Q. Shen, Flow of polymer and wormlike micellar solutions around microfluidic cylinders with low and high aspect ratio, Advances in Computational Fluid Structure Interaction and Flow Simulation (AFSI 2019), Okinawa, Japan, June 2019
- Amy Q. Shen, Novel glass microfluidic fabrication: new opportunities to probe flow instabilities of complex fluids, 9th International Colloids Conference, Spain, June 2019.
- Amy Q. Shen, New nano/microfluidic platforms for biosensing applications, Catalan Institute of Nanoscience and Nanotechnology, Autonomous University of Barcelona, Spain, June 2019.
- Amy Q. Shen, Glass microfluidic fabrication: new opportunities to probe flow instabilities and fluid-structure interactions, Department of Physics, University of Barcelona, Spain, June 2019.
- Amy Q. Shen, Microfluidic flows and instabilities of non-Newtonian fluids, The 30th Anniversary Symposium of the Korean Society of Rheology, Korea, May 2019.
- Amy Q. Shen, New observations of hydrodynamic instabilities around junctions by using microfluidics, Department of Biomedical Engineering, Korea University, May 2019.
- Amy Q. Shen, New observations of hydrodynamic instabilities around junctions by using microfluidics, Department of Chemical Engineering, University of Naples Federico II, Italy, April 2019.
- Amy Q. Shen, Flow instabilities in microfluidics, ICS-3 (Soft Matter), Forschungszentrum Juelich, Germany, April 2019.
- Amy Q. Shen, New nano/microfluidic platforms for biosensing applications, Institute of Complex Systems (ICS-8), Forschungszentrum Juelich, Germany, April 2019.
- Amy Q. Shen, New observations of hydrodynamic instabilities around junctions by using microfluidics, Department of structural and geotechnical engineering, Sapienza Università di Roma, Italy, April 2019.
- Chan, San To, Jesse Ault, Simon Haward, Eckart Meiburg, and Amy Shen., Coupling of vortex breakdown and stability in a vortex T-mixer flow, 72nd Annual Meeting of the APS Division of Fluid Dynamics, Seattle, Washington, USA, November 2019.
- Haward, Simon J., Hopkins, Cameron C., Shen, Amy Q., Viscoelastic fluid-structure interactions in microfluidics, 72nd American Physical Society Division of Fluid Dynamics Annual Meeting, Seattle, WA, November 2019.
- Shen, Amy Q., Burshtein, Noa, Haward, Simon J., Controlled symmetry breaking and vortex dynamics in intersecting flows, 72nd American Physical Society Division of Fluid Dynamics Annual Meeting, Seattle, WA, November 2019.
- Toda-Peters, Kazumi, Matsumoto, Atsushi, Shen Amy Q., Natto: the breakdown of capillary breakup, American Physical Society Division of Fluid Dynamics, Seattle, USA, November 2019.
- Atsushi Matsumoto, Francesco Del Giudice, Rachapun Ratrattanadumrong, and Amy Q. Shen, Rheological Scaling of Ionic Liquid-Based Polyelectrolytes in Ionic Liquid Solutions, Okinawa Colloids, Okinawa, Japan, November 2019.
- Sathish, Shivani, Toda-Peters, Kazumi and Shen, Amy Q, Integrated prototype for point-of-care diagnosis of Chlamydia trachomatis infections, 23rd International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS), Basel, Switzerland, October 2019.
- Hopkins, Cameron C., Haward, Simon J., Shen, Amy Q., Flow-induced vibrations of flexible microcylinders due to a viscoelastic flow instability, 91st Annual Meeting of the Society of Rheology, Raleigh, NC, USA, October 2019.
- Atsushi Matsumoto and Amy Q. Shen, Rheological Scaling of Imidazolium-Based Polyelectrolytes in Ionic Liquid Semidilute Solutions, Society of Rheology 91st Annual Meeting, Raleigh, North Carolina, USA, October 2019.
- Atsushi Matsumoto, Francesco Del Giudice, Rachapun Ratrattanadumrong, and Amy Q. Shen, Rheological Scaling of Ionic Liquid-Based Polyelectrolytes in Ionic Liquid Solutions, 67th Rheology Symposium of SRU, Shiga, Japan, October 2019.
- Varchanis, Stylianos, Haward, Simon J., Hopkins, Cameron C., Ioannou, G., Kordalis, A., Shen, Amy Q., Dimikopoulos, Yannis, Tsamopoulos, John, Elastic instabilities and nonlinear dynamics of yield stress fluids in cross-slot extensional rheometers, VPF8 Viscoplastic Fluids: from Theory to Application, Cambridge, UK, September 2019.
- Hopkins, Cameron C., Haward, Simon J., Shen, Amy Q. Flow-induced vibrations of flexible microcylinders due to a viscoelastic flow instability, First Rencontres du Vietnam – Soft Matter Science, Quy Nhon, Vitenam, July 2019.
- Haward, Simon J., Hopkins, Cameron C., Kitajima, Naoyuki, Toda-Peters, Kazumi, Takahashi, Tsutomo, Shen, Amy Q., Flow of wormlike micellar solutions around microfluidic cylinders with high aspect ratio and low blockage ratio, First Rencontres du Vietnam – Soft Matter Science, Quy Nhon, Vietnam, July 2019.
- Toda-Peters, Kazumi, Haward, Simon J., Shen, Amy Q., 3D Printed Glass: Novel Microfluidic Device Fabrication Using Selective Laser-induced Etching, Lab-on-a-chip and Microfluidics Europe, Rotterdam, Netherlands, June 2019.
- Tsai, Hsieh-Fu, Shen Amy Q., Usiigaci: Single cell segmentation and tracking in phase contrast microscopy using machine learning for directional cell migration analysis, Lab-on-a-Chip and Microfluidics Europe 2019, Rotterdam, Netherlands, June 2019.
- Haward, Simon J., Hopkins, Cameron C., Shen, Amy Q., Viscoelastic flow and instabilities around microfluidic cylinders, 19th International Workshop on Numerical Methods in Non-Newtonian Flows, Peso da Regua, Portugal, June, 2019.
- Atsushi Matsumoto, Francesco Del Giudice, Rachapun Ratrattanadumrong, and Amy Q. Shen, Rheological Scaling of Ionic Liquid-Based Polyelectrolytes in Ionic Liquid Solutions, 46th Annual Meeting of SRJ, Kyoto, Japan, May 2019.
- Burshtein, Noa, Zografos, Konstantinos, Shen, Amy Q., Poole, Robert J., Haward, Simon J., Inertioelastic effects on a spiral vortex flow instability, Annual European Rheology Conference, Portoroz, Slovenia, April 2019.
- Haward, Simon J., Kitajima, Naoyuki, Toda-Peters, Kazumi, Takahashi, Tsutomo, Shen, Amy Q., Flow of wormlike micellar solutions around microfluidic cylinders with high aspect ratio and low blockage ratio, Annual European Rheology Conference, Portoroz, Slovenia, April 2019.
- Haward, Simon J., Page, Jacob, Zaki, Tamer A., Shen, Amy Q., Experimental investigation of viscoelastic effects in wavy microchannel flow, Annual European Rheology Conference, Portoroz, Slovenia, April 2019.
- Atsushi Matsumoto, Francesco Del Giudice, Rachapun Ratrattanadumrong, and Amy Q. Shen, Rheological Scaling of Ionic Liquid-Based Polyelectrolytes in Ionic Liquid Solutions, Annual European Rheology Conference 2019, Portoroz, Slovenia, April 2019.
- Haward, Simon J., Microfluidic Flows and Instabilities of Wormlike Micellar Solutions Around Cylinders. Lindner Group Seminar, ESPCI, Paris, France, April 2019.
5. Intellectual Property Rights and Other Specific Achievements
5.1 Patents:
1. H.-F. Tsai and Amy Q. Shen, “A detachable microfluidic system and method for robust sparse cell seeding without bubbles”, US application 62/968,768, January 2020.
5.2 POC: Portable nanoplasmonic instrumentation (NANOCUBE)
The OIST Proof of Concept (POC) Program is an internal, competitive funding program that is designed to support targeted research with high potential for developing innovative technologies, and to help bridge the technical and funding gap between lab discoveries and commercialization. We are developing a portable instrument based on the localized surface plasmon resonance (LSPR) sensing technique we patented in 2017. The proposed system will be used as a generic biosensing platform for a wide range of personal care, health, food, and environment monitoring by detecting the binding events in a bio/chemical assay. Our sytems have key advantages over existing technologies, such as fast turnaround time of diagnosis, high sensitivity & selectivity of the measured analyte, and cost-effectiveness. See the promotion video here.
5.3 Grants and Fellowships:
- Amy Shen (PI); Oct 2019--Sep 2022; JPY 30,000,000; JSPS and SNSF under the Joint Research Projects (JRPs). Scale-dependent active microrheology of soft materials by studying driven motion of microbeads.
- Amy Shen (co-PI); Apr 2018–Mar 2022; JPY 3,000,000 out of JPY 13,000,000 total; Grants-in-Aid for Scientific Research (B), #18H01135, Japan. Mathematical modeling of vortices formed in viscoelastic fluids; Hirofumi Notsu from Kanazawa University (PI).
- Simon Haward (PI); Apr 2018–Mar 2021; JPY 3,300,000; Grants-in-Aid for Scientific Research (C), #18K03958, Japan. Wagging the tail: Elasticity and flexible filaments in microscopic flows.
- Atsushi Matsumoto (PI); Apr 2019 – Mar 2021; JPY 2,860,000; Grants-in-Aid for Early-Career Scientists, #19K15641, Japan. Elucidating the Unusual Charge Screening Effect on the Polymer Dynamics of Polymerized Ionic Liquids.
- Shivani Sathish, Apr 2019– Mar 2021; JPY 2,400,000; JSPS Research Fellowship (DC2), Japan.
6. Meetings and Events
6.1 Seminars
1. Prof. Arben Mercoci (CSIC and The Barcelona Institute of Science and Technology, Spain)
- Date: April 17, 2019
- Venue: OIST Campus, D014
- Seminar: Nanomaterial-based devices for diagnostics applications
2. Prof. Gregory Jedd (TEMASEK Life Science Laboratory)
- Date: May 30, 2019
- Venue: OIST Campus, D015
- Seminar: Deterministic roles for cytoplasmic flow in cellular decision-making
3. Dr. Jonathan Wylie (City University of Hong Kong)
- Date: June 5, 2019
- Venue: OIST Campus, C016
- Seminar: Stretching of viscous threads
4. Prof. Guozhong Cao (University of Washington)
- Date: July 24, 2019
- Venue: OIST Campus, C016
- Seminar: From lithium to zinc-ion batteries: electroactive cathode materials
5. Mr. Vincenzo Calabrese (University of Bath)
- Date: July 30, 2019
- Venue: OIST Campus, D015
- Seminar: Nanocellulose assembly in aqueous media: towards functional hybrid materials
6. Mr. Peter Gilbert (Queen's University)
- Date: August 14, 2019
- Venue: OIST Campus, C015
- Seminar: Illuminating Complex Fluid Flow
7. Prof. Timm Krueger (University of Edinburgh)
- Date: September 25, 2019
- Venue: OIST Campus, D015
- Seminar: Modelling of in-vivo blood flow and in-vitro microfluidics for healthcare applications
8. Ms. Charlotte de Blois (ESPCI Paris)
- Date: September 26, 2019
- Venue: OIST Campus, D015
- Seminar: Swimming water droplet in complex environment, confinement, gravity and collective effects
9. Prof. Pavlik Lettinga (Forschungszentrum Juelich GmbH)
- Date: November 1, 2019
- Venue: OIST Campus, D015
- Seminar: The link between flow instabilities and microstructure for filamentous particles of variable stiffness
10. Prof. Jean-Francois Berret (CNRS (Centre national de la recherche scientifique))
- Date: November 11, 2019
- Venue: OIST Campus, D015
- Seminar: Magnetic wires for active micro-rheology: applications to intra- and extra-cellular body fluids
11. Mr. Hugh Barlow (University of Durham)
- Date: November 13, 2019
- Venue: OIST Campus, C016
- Seminar: Yielding and transient shear-banding in soft glassy materials
12. Prof. Kyongok Kang (Forschungszentrum Juelich)
- Date: December 3, 2019
- Venue: OIST Campus, D015
- Seminar: Protein Diffusion and Kinetics in Low Electric-field Frequency Modulations
13. Prof. Noritada Kaji (Kyushu University), Prof. Tatsuro Endo (Osaka Prefecture University), Prof. Mao Fukuyama (Tohoku University) and Prof. Satoshi Yamaguchi (The University of Tokyo)
- Date: January 20, 2020
- Venue: OIST Campus, D014
- Seminar: "Mechanical phenotyping of a single cell by a microfluidic device", "Functionalized polymer-based photonic crystal nanocavity for biosensing application", "Control of the molecular transfer between microdroplets and micelles" and "Photo-responsive cell immobilization tools for single-cell manipulation"
6.2 Events
OIST Mini Symposium "Fluid-Structure Interactions: From Engineering to Biomimetic Systems"
7. Other
7.1 Events at OIST
7.1.1. OIST Skill Pills
Ph.D student Ainash Garifullina gave LateX Skill Pills on November and December 2019.
7.1.2. World Ocean Day outreach event
Ph.D student Ainash Garifullina coordinated this event on June 2019.
7.2 Community Outreach Activities
7.2.1. OIST-wide fundraise event
Ph.D student Shivani Sathish Organized an OIST-wide fundraiser event to collect donations for wildlife rehabilitation in Australia. A total of 46,000 yen was collected and donated to World Wildlife Fund (WWF) and WIRES, Australia, on behalf of OIST.
7.2.2. Pacific Region Junior Science and Humanities Symposium
Ph.D students Shivani Sathish and Ainash Garifullina mentored high school students from Nile C. Kinnick High School, Yokosuka, Japan, as part of the Pacific Region Junior Science and Humanities Symposium, organized by the American Department of Defense Education Activity (DoDEA). The student mentored by Shivani presented a research paper elucidating the effects of handwashing time on the growth and transfer of bacteria between people. His project was selected to be presented at the competitive Pacific Far East regional competition, on March 16th, 2020.
7.2.3 Monthly Nerd Nite Okinawa
Ph.D student Ainash Garifullina coordinated this monthly event by identifying speakers and working with them to make sure their talks are aligned with the general theme of the event with broad appeals.


7.2.4 Oki’s Omocha for Orphans
Ph.D student Ainash Garifullina coordinated the 3rd annual Christmas toy drive for local orphans.
7.2.5 International Food Festival and Open TIDA Market
Ph.D student Ainash Garifullina was in charge of collecting donations for HelpOki & Hearts Charity Organization.
















