Seminar "Translational control by poly(A)-binding protein during glucose-stimulated insulin biosynthesis in mouse pancreatic islets"
Date
Location
Description
Speaker: Dr. Akiko Yanagiya
Goodman Cancer Research Centre, McGill University, Montreal, Quebec, CANADA
Title: Translational control by poly(A)-binding protein during glucose-stimulated insulin biosynthesis in mouse pancreatic islets
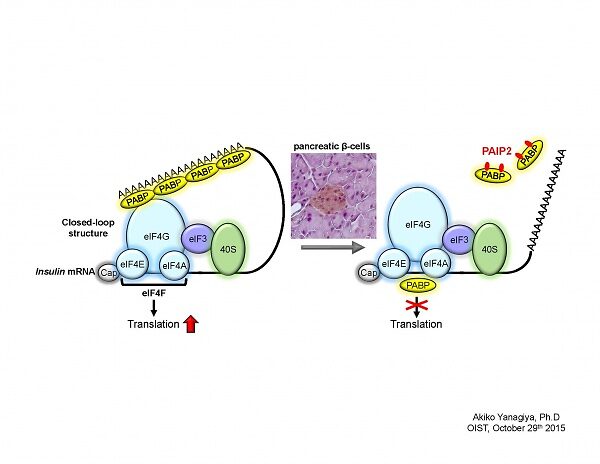
Abstract: Translational control plays an important role in gene expression in response to extracellular stimuli and/or during certain periods of cell differentiation in which transcriptional regulation is suppressed. Translation initiation requires the recruitment of the ribosome to the 5’ end of mRNA by eukaryotic translation initiation factor 4F complex (eIF4F). All eukaryotic cellular mRNA processes a poly(A) tail at the 3’ end, and the interaction between poly (A)-binding protein (PABP) and eIF4F enables mRNA to form the closed-loop structure, leading to translational activation by the recycling of ribosomes.
PABP plays a key role in translational control by regulating translational efficiency and mRNA stability. PABP activity is modulated by PABP-interacting proteins (PAIPs), PAIP1 and PAIP2. PAIP2 disrupts the closed loop structure of mRNA by releasing PABP from the mRNA poly(A) tail, leading to translational inhibition.
Glucose-stimulated insulin biosynthesis in pancreatic β-cells is under translational control without any change of insulin mRNA levels. Previous studies have indicated that some RNA-binding proteins that associate with the 5’ untranslated region (5’UTR) of insulin mRNA regulate translation of insulin mRNA in response to increased blood glucose, but the molecular mechanism underlying this phenomenon remains obscure.
Intriguingly, glucose-stimulated insulin biosynthesis is impaired in pancreatic β-cells of Paip2a-knockout (KO) mice. Moreover, PABP protein level is decreased at high glucose, implying a role of PABP in translational regulation of insulin mRNA.
In this study, we demonstrate that the interaction between insulin mRNA and PABP is reduced during glucose-stimulated insulin biosynthesis in mouse pancreatic islets. PABP is proteolytically cleaved by a calcium-dependent protease, calpain, in response to elevated glucose. Although PABP is a positive regulator of translation, we have previously shown that excess amount of PABP inhibits translation by interacting with the 5’UTR of mRNA, thereby interfering with the association between the cap structure of mRNA and the cap-binding complex, eIF4F. Hence, decreased PABP association to insulin mRNA contributes to increased translation of insulin mRNA triggered by elevated glucose in mouse pancreatic islets.
Here, I demonstrate a novel coordinated molecular mechanism exerted by PABP and PAIP2A during glucose-stimulated insulin biosynthesis, in which PAIP2A attenuates the inhibitory effect of excess PABP that associates not only with the poly(A) tail but also with the 5’UTR of insulin mRNA in glucose-stimulated insulin biosynthesis.
Host: Tadashi Yamamoto, Cell Signal Unit
Subscribe to the OIST Calendar: Right-click to download, then open in your calendar application.



