Seminar "Identification of a novel p53 downstream pathway important in neuroendocrine tumor development."
Date
Location
Description
Speaker: Rieko Ohki
Laboratory of Fundamental Oncology, National Cancer Center Research Institute, Tokyo, Japan
Title: Identification of a novel p53 downstream pathway important in neuroendocrine tumor development.
Abstract: The most important function of p53 is the transcriptional regulation of p53 target genes. Although many p53 target genes have been identified to date, further studies identifying novel p53 target genes may lead to discovery of novel tumor-associated genes, and will provide more advanced understanding of the complex regulation of death, survival and proliferation.
We have performed an exhaustive screen of p53-inducible genes by microarray expression analysis and of genome-wide p53 binding sites by ChIP-chip analysis. By combining the results of both analyses, we were able to identify a number of candidate p53 target genes. Among these candidates, we are currently analyzing genes that are potentially important for tumor suppression, focusing on genes that are: 1) strictly regulated by p53, and 2) of unknown function. Among these, we have characterized and reported the molecular function of several novel p53 target genes, including Noxa, Reprimo, AEN, FUCA1, IER5 and PHLDA3. These genes are involved in fundamental tumor suppression pathways; including apoptosis, cell cycle, proliferation and stress response.
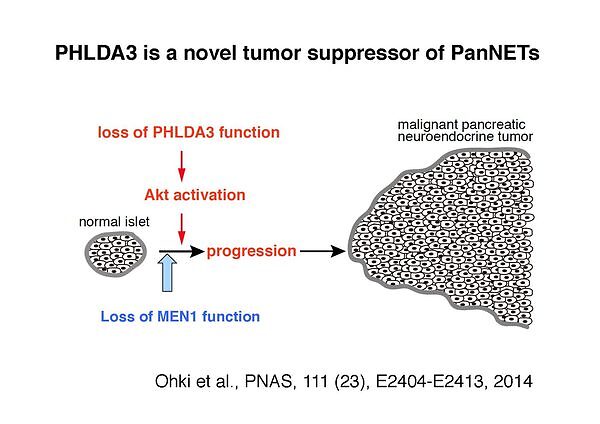
Here, we report on PHLDA3, a tumor suppressor gene encoding a repressor of Akt. PHLDA3 appears to function as a dominant negative regulator of Akt, suppressing Akt translocation to the cellular membrane and its subsequent phosphorylation/activation (Cell, 136, 535-550, 2009). We have also shown that PHLDA3 can function as a tumor suppressor gene in various neuroendocrine tumors (NETs). The genomic region of the PHLDA3 gene undergoes a loss of heterozygosity (LOH) at a remarkably high frequency in various NETs. In addition to LOH, the PHLDA3 gene undergoes mutation or methylation, suggesting that a 2-hit inactivation of the PHLDA3 gene is required for NET development (PNAS, 111, E2404-E2413, 2014, and unpublished data). Furthermore, loss of PHLDA3 function in NETs is mutually exclusive with loss of p53 function, suggesting that PHLDA3 is a pivotal downstream mediator of p53 in NET suppression. We also demonstrate that PHLDA3 represses Akt activity and Akt-regulated biological processes in endocrine tissues, and that PHLDA3-deficient mice develop abnormalities in endocrine tissues. Collectively, these results indicate the existence of a novel p53-PHLDA3-mediated pathway of tumor suppression that is important for the development of NETs.
Reference
1. Rieko Ohki#, Kozue Saito, Yu Chen, Tatsuya Kawase3, Yukie Aita, Nobuyoshi Hiraoka, Raira Saigawa, Maiko Minegishi, Goichi Yanai, Hiroko Shimizu, Shinichi Yachida, Naoaki Sakata, Akihiko Yokoyama, Ryuichiro Doi, Tomoo Kosuge, Kazuaki Shimada, Benjamin Tycko, Toshihiko Tsukada, Yae Kanai, Shoichiro Sumi, Hideo Namiki, Yoichi Taya, Tatsuhiro Shibata and Hitoshi Nakagama. (#corresponding author) PHLDA3 is a novel tumor suppressor of pancreatic neuroendocrine tumors.
Proc Natl Acad Sci USA, 111 (23), E2404-E2413, 2014.
2. Tatsuya Kawase*, Rieko Ohki#, *, Tatsuhiro Shibata, Shuichi Tsutsumi, Naoko Kamimura, Johji Inazawa, Tsutomu Ohta, Hitoshi Ichikawa, Hiroyuki Aburatani, Fumio Tashiro and Yoichi Taya. (*These authors equally contributed to the work, #corresponding author) PH domain-only protein PHLDA3 is a p53-regulated repressor of Akt.
Cell, Vol. 136, pp. 535-550, 2009.
Subscribe to the OIST Calendar: Right-click to download, then open in your calendar application.



