FY2011 Annual Report
Developmental Neurobiology Unit
Professor Ichiro Masai
Research Theme: Mechanism underlying neuronal differentiation and degeneration in the zebrafish retina

Abstract
The vertebrate neural retina is derived from the ventral region of the forebrain. In this region, six classes of neurons differentiate and form the neural circuit underlying visual transduction. Thus, the retina provides an excellent model for studying cell differentiation and neural circuit formation in the vertebrate brain. Furthermore, more than one hundred hereditary retinal diseases causing photoreceptor degeneration have been identified in humans. Understanding the pathological processes of photoreceptor degeneration is an important issue from a medical perspective. We are currently investigating the mechanisms underlying retinal cell differentiation and photoreceptor degeneration, using zebrafish as an animal model. The results have revealed that five signaling molecules, including Histone deacetylase 1 (Hdac1), regulate retinal neurogenesis in zebrafish. Regulatory factors that modulate the Hdac1 activity during retinal neurogenesis have been examined. Furthermore, to examine cell-cycle regulation in retinal neurogenesis with high accuracy, we visualized cell-cycle phases differentially with fluorescent proteins using zebrafish fluorescent ubiquitination-based cell cycle indicator (Fucci). In 2011, we applied this Fucci technique to zebrafish lens epithelium and revealed a dynamic regulation of cell proliferation and division. In the project on photoreceptor degeneration, we have identified zebrafish mutants showing photoreceptor degeneration. In 2011, we revealed that photoreceptors undergo apoptosis in a zebrafish b-SNAP mutant. Because b-SNAP regulates the fusion of transport vesicles to intracellular membrane organelles, this finding suggests that vesicular fusion defects cause photoreceptor degeneration.
1. Staff
- Dr. Yuko Nishiwaki, Researcher
- Dr. Toshiaki Mochizuki, Researcher
- Dr. Junichi Kawada, Researcher
- Dr. Ekramul Islam, Researcher
- Ms. Asuka Yoshizawa, Technician
- Mr. Yutaka Kojima, Technician
- Mr. Shohei Nakamura, Technician
- Ms. Eri Oguri (temporal worker), Technician
- Ms. Yayoi Tomoyose, Research Assistant
- Ms. Ayako Nameki, Research Assistant
- Ms. Kazumi Toguchi, Research Assistant
- Dr. Mariko Kinoshita-Kawada, Research Assistant
- Ms Ayako Gima, Research Administrator/Secretary
2. Collaborations
- Theme: In vivo functional analysis of hypoxia response genes using the zebrafish retina
- Type of collaboration: Joint research agreement
- Researchers: Dr. Ichiro Masai (Developmental neurobiology unit, OIST), Dr. Masayuki Matsushita (Department of Medicine, Ryukyu University)
3. Activities and Findings
3.1 Mechanism underlying retinal neurogenesis in zebrafish
The vertebrate retina is initially specified in the anterior neural plate and evaginates from the ventral region of the forebrain as the optic cup. In this region, six major classes of retinal neurons and one class of glial cells differentiate to form the neural circuit that mediates phototransduction and visual processing (Fig. 1). Thus, the retina provides an excellent model for studying the mechanisms underlying cell differentiation and neural circuit formation in the developing brain.In the developing zebrafish retina, neurogenesis is initiated at a few cells adjacent to the optic stalk and progresses to the entire neural retina. This pattern of retinal neurogenesis provides a good model for studying the mechanisms regulating the spatial and the temporal pattern of neurogenesis in the nervous system. Previous studies, including research in our lab, suggest that at least five families of signaling molecules regulate the pattern of retinal neurogenesis in zebrafish (Fig. 2). Fibroblast growth factors (Fgfs) are expressed in the optic stalk and are required for the initial induction of retinal neurogenesis in zebrafish. The Hedgehog (Hh) signaling pathway is important for the progression of retinal neurogenesis in zebrafish. Activation of the Wnt signaling pathway promotes cell proliferation in the zebrafish retina, and activation of the Notch pathway inhibits retinal neurogenesis. Previously, we identified a zebrafish histone deacetylase 1 (hadc1) mutant, in which retinal progenitor cells fail to exit from the cell cycle and continue to proliferate. HDAC1 promotes retinal neurogenesis by suppressing both the Wnt and Notch signaling pathways. HDAC1 is recruited to several transcription repressor and corepressor complexes as well as those in the Wnt and Notch pathways. To elucidate the HDAC1-dependent regulation of retinal neurogenesis, we have identified a new mutation, oki150, that modifies the neurogenic defects in heterozygous hdac1 mutants. In 2011, using polymorphic markers, we mapped the oki150 mutational locus on zebrafish chromosome 20.
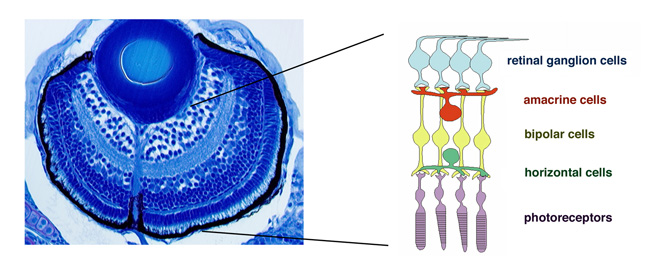
Figure 1: Zebrafish retina. Plastic section of 3 dpf zebrafish retina. Six major classes of retinal neuron including photoreceptors differentiate and form layered structures.
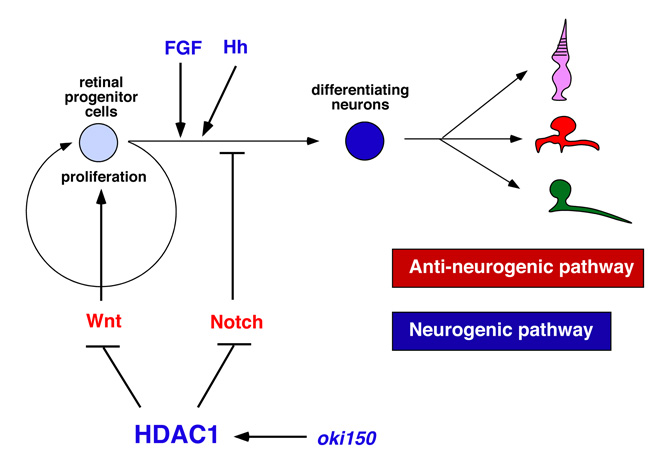
Figure 2: Molecular signaling network regulating retinal neurogenesis in zebrafish. FGF and Hh regulate the induction and progression of retinal neurogenesis in zebrafish. Wnt and Notch signaling pathways promote cell differentiation and neurogenic inhibition, respectively. Hdac1 promotes retinal neurogenesis by suppressing both Wnt and Notch signaling pathways. A novel mutation namely oki150 interacts with Hdac1 to promote neurogenesis.
3.2 Visualization of cell-cycle phases with fluorescent proteins using Fucci
The first step of neurogenesis is the exit from the cell cycle. Previously we showed that the earliest neurogenic marker, ath5 (also designated as atoh7), is initiated in the G2 phase in retinal progenitor cells just prior to the final cell division, which generates two neuronal daughter cells. Thus, it is very likely that the commitment to the retinal neuron is associated with cell-cycle progression. To accurately understand the relationship between cell proliferation and neurogenesis, we visualized cell-cycle phases differentially with fluorescent proteins, using the zebrafish Fucci (fluorescent ubiquitination-based cell cycle indicator) (Sugiyama et al., 2009, Proc Natl Acad Sci USA 106, 20812–20817). In the Fucci system, the cell-cycle dependent expression of two cell-cycle regulators, Geminin and Cdt1, are visualized with fluorescent proteins fused to their regulatory peptide sequences. We generated the zebrafish transgenic line carrying genes encoding mCherry-tagged Geminin and green fluorescent protein (GFP)-tagged Histone 2A. In this transgenic fish, we were able to clearly distinguish three different phases in the developing retina as well as the lens; the G1, S-G2, and M phases (Fig. 3A). Furthermore, we were able to observe chromosome condensation in the M phase (Fig. 3B) and measure the orientation of cell division from in vivo time-lapse imaging data.
Next, using this transgenic fish, we observed the cell-cycle progression and cell division in the zebrafish lens epithelium using time-lapse techniques, because the lens epithelium is more easily accessed than the neural retina (Fig. 4A). We found that cell proliferation was highly active in a marginal zone just anterior to the equator in the lens epithelium. Furthermore, cell-division orientation was biased along the circumferential axis in this proliferating zone (Fig. 4B). These data suggest that cell proliferation and cell division are dynamically regulated in the zebrafish lens epithelium. In many developing tissues, it has been reported that cells tend to divide along their longest axis (Fig. 4C). This is referred to as “the Hertwig rule”. We found that the longest axis was also circumferentially biased in the proliferating zone in the zebrafish lens epithelium (Fig. 4D), suggesting that lens epithelial cells follow the Hertwig rule. These observations suggest that apical cell geometry correlates with cell division orientation in the proliferating zone of the lens epithelium (Fig. 4E).
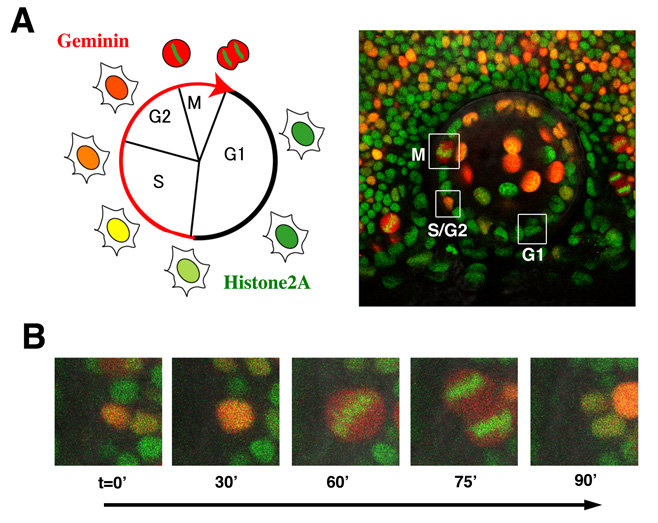
Figure 3: Visualization of cell-cycle progression in the zebrafish retina and lens. (A) (Left panel) Relationship between cell cycle phases and fluorescent protein expression. Geminin (red) are expressed exclusively in S/G2/M phase. GFP-tagged Histone2A (green) labels nuclei. (Right image) Confocal images of zebrafish transgenic line carrying GFP-tagged Histone 2A (green) and mCherry-tagged Geminin (red). Cells in the G1, S/G2, and M phase are distinguished. (B) Confocal images of mitosis in zebrafish retinal cells line with GFP-tagged Histone 2A (green) and mCherry-tagged Geminin (red) transgenes. GFP-tagged Histone2A expression (green) becomes condensed in segregating chromosomes.
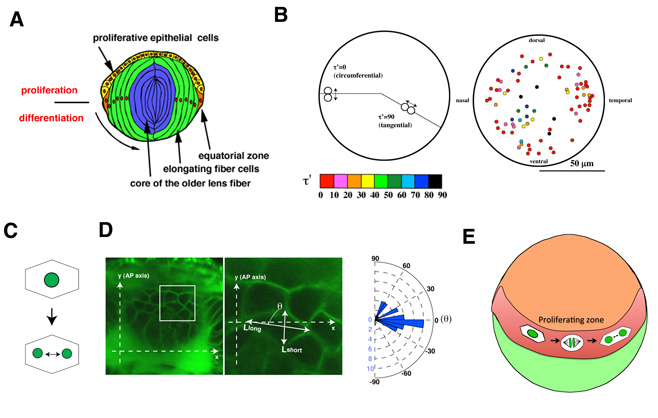
Figure 4: Cell proliferation and division are spatially regulated in the zebrafish lens. (A) Schematic drawing of lens structures. Anterior lens epithelial cells (yellow) are proliferative and start to differentiate into lens fiber cells (green) at the equator (orange). Differentiating lens fiber cells elongate to cover the old lens fiber core (blue). (B) Plotting of mitosis position on anterior projection view of lens epithelium. Cell division was more frequent in the marginal zone anterior to the equator. Angles of cell-division orientation relative to the circumferential axis are indicated with color tone spectrum from red (0 degree) to blue (90 degree). Cell division was the most frequent in the marginal zone anterior to the equator and its orientation was biased circumferentially. (C) The Hertwig rule. Cells tend to divide along their longest axis. (D) (Left) Labeling of lens epithelium at the proliferating zone with a fluorescent lipid dye (green), which visualizes cell membranes. (Right) Histogram of angle of cell division to the circumferential axis. A majority of cell divisions are in parallel with the circumferential axis. (E) Schematic drawing of cell division of zebrafish lens epithelium. Cell proliferation is highly active in the peripheral zone (red) of zebrafish lens epithelium (orange). In this proliferating zone, lens epithelial cells are elongated along the circumferential axis, which may result in the circumferential-oriented cell division.
3.3 Mechanism underlying axonal guidance crossing the midline
Visual information processed in the retina is transmitted from retinal ganglion cells to the second visual center, called the optic tectum, through retinal axons. In lower vertebrates including fish, all retinal axons project to the contra-lateral side of optic tectum and no ipsi-lateral projection occurs, resulting in the formation of the optic chiasm, where retinal axons from the left and right eyes cross without combining. Our previous study showed that retinal axons are misrouted to the ipsi-lateral side of the optic in zebrafish N-cadherin mutants (Fig. 5A), suggesting a role of cell adhesion in retinal axon guidance at the midline. However, the mechanism underlying retinal axon guidance in crossing on the midline remains to be elucidated.
The molecular mechanism underlying axon guidance at the midline has been studied using commissural neurons in the vertebrate hindbrain and spinal cord as well as in the Drosophila embryonic central nervous system (CNS) (Fig. 5B). It is generally accepted that pre-crossing axons are attracted by a guidance cue emanating from the midline tissue, Netrin, and that the midline tissue expresses not only Netrin but also a repellent cue, Slit. A recent model proposes that pre-crossing axons are insensitive to Slit and only respond to Netrin, resulting in the attraction of their growth cone to the midline. When their growth cone passes over the midline, the sensitivity to Slit is increased, which enables post-crossing axons to leave from the midline. It has been reported that the expression level of a receptor of Slit, namely Roundabout (Robo), is very low in pre-crossing axons but increased in post-crossing axons in Drosophila CNS. In addition, it was reported that one of the Robo family proteins, Robo3, is exclusively expressed in the pre-crossing axons and functions as dominant-negative repressor of Robo1, which is thought to mediate repulsive response instructed by Slit. However, the way in which the responsiveness of Slit is differentially regulated in pre- and post-crossing axons remains unclear. We have established an in vitro culture assay system using commissural axons that reflects the acquisition of Slit responsiveness in post-crossing axons. Using this assay system, we showed that a Robo1-associating deubiquitinase, USP33, is required for the acquisition of Slit responsiveness and crossing of commissural axons over the midline. In 2011, we further revealed that the endocytic trafficking of Robo1 modulates its own expression level on axons as well as the sensitivity of axons to Slit. These findings suggest a new mechanism that modulates axonal responsiveness to guidance cues in crossing the midline of the CNS.
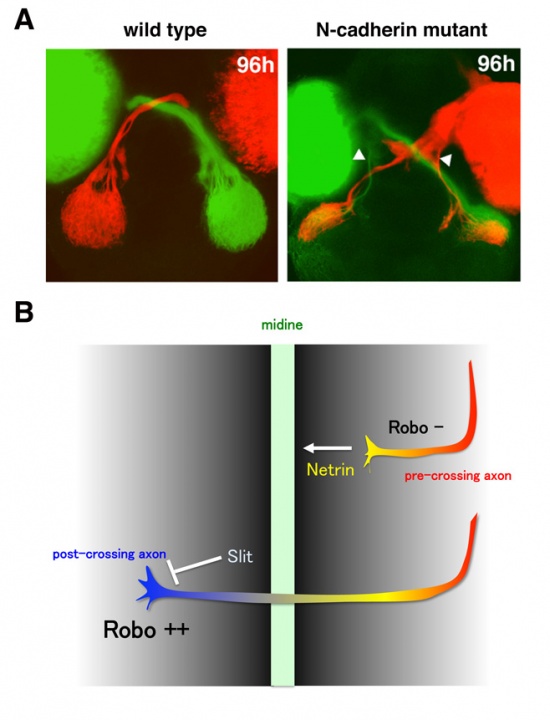
Figure 5: Axon guidance at the midline. (A) Labeling of zebrafish retinal axons with diI (red) and diO (green). Retinal axons from originated from the right (red) and left eye (green) cross without combining each other to project into the contra-lateral optic tectum in wild type. However, some of retinal axons are misrouted to the ipsi-lateral optic tectum in the N-cadherin mutant, suggesting a role of cell adhesion in axon guidance at the midline. (B) Model on the mechanism underlying axon guidance at the midline. Two molecules, Netrin and Slit, are secreted from the midline and function as attractant and repulsive guidance cues, respectively. Because pre-crossing axons lack the Slit receptor Robo, they extend toward the midline in response to Netrin. On the other hand, post-crossing axons express Robo and leave from the midline in response to Slit. However, the mechanism underlying the upregulation of Robo in post-crossing axons remains unclear.
3.4 Mechanisms underlying photoreceptor degeneration
Photoreceptors are highly specialized neurons that efficiently detect light stimuli. To date, more than a hundred genes associated with inherited photoreceptor degeneration have been identified in humans (see the homepage of the Retinal Information Network at http://www.sph.uth.tmc.edu/Retnet/). Photoreceptor degeneration is unique in that mutations in genes encoding proteins of great functional diversity lead to the death of cells, for example proteins involved in phototransduction, retinol metabolism and the transport of proteins to the photoreceptive membrane through intraflagellar transport. However, the mechanisms underlying the pathological processes involved in photoreceptor degeneration also remain to be elucidated.
To determine the mechanisms underlying photoreceptor degeneration, we have screened zebrafish mutants with dysfunctional visual behavior by testing the optokinetic response. We identified several zebrafish mutants, in which photoreceptors differentiate but later degenerate. In 2010, we reported that one of these mutants carried a genetic mutation in cGMP-phosphodiesterase 6c (PDE6c), a mediator molecule of cone-specific phototransduction, suggesting a link between achromatopsia and progressive cone dystrophy. In 2011, we focused on another mutant, coa, whose photoreceptors rapidly undergo degeneration before maturation (Fig. 6A). We found that the coa mutant gene encodes b-soluble N-ethylmaleimide-sensitive factor attachment protein (b-SNAP). b-SNAP regulates the fusion of transport vesicles to intracellular membrane organelles. We consistently observed that the coa mutant showed defects in photoreceptive protein transport. These observations suggest that vesicular transport defects cause photoreceptor degeneration in zebrafish. Mitochondria-dependent apoptosis is promoted by proapoptotic Bcl2 family proteins, Bax, and inhibited by anti-apoptotic Bcl2 family proteins, Bcl2. We found that overexpression of Bcl2 or inhibition of Bax rescued photoreceptor degeneration in the coa mutant (Fig. 6B), suggesting that their photoreceptors undergo Bax-dependent apoptosis. It is generally accepted that BH3-only proteins promote apoptosis by modulating the balance between pro-apoptotic and anti-apoptotic Bcl2 proteins. Previously we showed that the activation of a tumor suppressor p53 induces severe apoptosis of retinal cells in zebrafish. However, we found that the inhibition of p53 failed to suppress photoreceptor apoptosis in the coa mutant, suggesting that photoreceptor apoptosis in the coa mutant is independent of p53 and possibly its downstream BH3-only protein, PUMA (Fig. 6C). To understand the molecular mechanism linking vesicular transport defects and apoptosis, we are currently investigating which BH3-only proteins are involved in photoreceptor degeneration in the coa mutant.
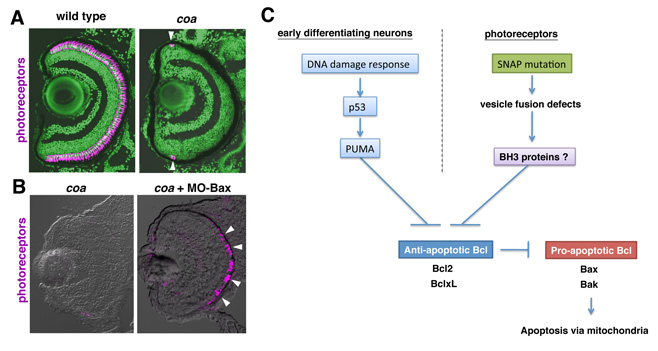
Figure 6: Photoreceptors undergo Bax-dependent apoptosis in zebrafish b-SNAP mutant. (A) Labeling of wild-type and coa mutant retinas with the zpr1 antibody, which stains photoreceptors (magenta). Photoreceptors are absent in the coa mutant, except in the ciliary marginal zone, where retinal stem cells continue to produce neurons (arrowheads), suggesting that photoreceptors undergo degeneration before maturation. (B) Labeling of retinas of coa mutant, and coa mutant injected with MO-Bax. MO-Bax restores photoreceptor survival in the coa mutant (arrowheads). (C) b-SNAP mutation induces Bax-dependent photoreceptor apoptosis in zebrafish. We showed that DNA damage response activates p53-dependent retinal apoptosis in zebrafish. However, photoreceptor apoptosis in the coa mutant is independent of p53 and possibly its downstream BH3-only protein PUMA, suggesting a novel BH3-only protein involved in b-SNAP mutation-dependent apoptosis.
4. Publications
4.1 Journals
-
Suzuki, E., Masai, I., Inoue, H. Phosphoinositide metabolism in Drosophila phototransduction: a coffee break discussion leads to 30 years of history. J Neurogenet 26, 34-42. (2012).
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Masai, I. Molecular mechanism that transforms defects in intracellular vesicular transport into apoptosis in zebrafish photoreceptors. 5th Asia-Oceania zebrafish international meeting, China National Convention Center, Beijing, China, 26-29 August 2011.
- Masai, I. Retinal cell fate determination. Developmental Neurobiology Course 2011, OIST seaside house, 17-31 July 2011.
- Mochizuki, T., Suzuki, S., Sakaue-Sawano, A., Miyawaki, A., Masai, I. Visualization of cell cycle and cell division in lens using Geminin-mCherry/H2A-GFP transgenic zebrafish. 44th Annual Meeting of the Japanese Society of Developmental Biologists, Okinawa Convention Center (Ginowan City), 18-21 May 2011.
- Mochizuki, T., Suzuki, S., Sakaue-Sawano, A., Miyawaki, A., Masai, I. Visualization of cell cycle and cell division in lens using Geminin-mCherry/H2A-GFP transgenic zebrafish. 17th Japanese Medaka and Zebrafish Meeting, Toray Human Resources Development Center, Mishima, Japan, 8-9 September, 2011.
- Mochizuki, T., Suzuki, S., Sakaue-Sawano, A., Miyawaki, A., Masai, I. Visualization of cell cycle and cell division in lens using Geminin-mCherry/H2A-GFP transgenic zebrafish., 34th Annual Meeting of the Molecular Biology Society of Japan, Pcifico Yokohama, Yokohama, Japan, 13-16 December, 2011.
- Nishiwaki, Y., Suzuki, S., Yoshizawa, A., Kojima, Y., Mochizuki, T., Masai, I. A zebrafish corona mutation induces a Bcl2-dependent apoptosis in photoreceptor. 44th Annual Meeting of the Japanese Society of Developmental Biologists, Okinawa Convention Center (Ginowan City), 18-21 May 2011.
- Nishiwaki, Y., Suzuki, S., Yoshizawa, A., Kojima, Y., Mochizuki, T., Masai, I. Mutation of beta-SNAP induces a mitochondria-dependent apoptosis in zebrafish photoreceptors. 7th European Zebrafish Meeting, Edinburgh, Scotland, 5-9 June 2011.
- Masai, I. Mechanism underlying neuronal degeneration using zebrafish retina as a model. Kansai Ganka Sensin Iryou Kenkyuukai, Osaka University, 1 February 2012. (in Japanese)
- Mochizuki, T. Visualization of cell cycle and cell division in lens using zebrafish tarnsgenic line carrying mCherry/H2A-GFP. 4th Retina research Meeting, Institute of Medical Reseach, University of Tokyo, 12 November 2011 (in Japanese)
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 OIST course
- Title: Developmental Neurobiology Course 2011
- Co-organizer: Mary Ann Price (OIST), David L. van Vactor (Harvard Medical School, OIST), Robert Baughman (OIST), Akinao Nose (University of Tokyo)
- Dates: 17-31 July 2011
- Place: OIST Seaside House
- Speakers: David L. van Vactor (Harvard Medical School, OIST), Mary Ann Price (OIST), James Brisco (NIMR, UK), Chris Q. Doe (University of Oregon), Ichiro Masai (OIST), Kozo Kaibuchi (Nagoya University), Masayuki Miura (University of Tokyo), Elke Stein (Yale University), Hiroshi Sakano (University of Tokyo), Z. Josh Huang (Cold Spring Harbor Lab), Akinao Nose (University of Tokyo), Yuh Nung Jan (UCSF), K. VijayRaghavan (Tata Institute of Fundamental Research), Hitoshi Okamoto (RIKEN BSI), David A. Feldheim (UC, Santa Cruz), Bernaerdo L. Sabatini (Harvard University), Takeharu Nagai (Hokkaido University), Hideyuki Okano (Keio University), Lee L. Rubin (Harvard University)
6.2 Seminar
- Title: A different pattern of alternative splice forms of USH1C and USH1F between the mouse and human retina
- Date: 22 February 2012
- Place: OIST campus
- Speakers: Dr. Maria Iribarne (Genetics and Physiology of Hearing Group, Pasteur Institute)
7. Other
7.1 Conference
- Title: 44th Annual meeting of the Japanese society of Developmental Biologists
- Dates: 18-21 May 2011
- Venue: Okinawa Convention Center (Ginowan city, Okinawa)
- Chief organizer: Ichiro Masai (OIST)
- Co-organizer: Shigeo Hayashi (RIKEN CDB), Toshihiko Fujimori (NIBB), Hiroshi Wada (Tsukuba University), Shinichi Nakagawa (RIKEN)
- Remark: This conference was held as an international conference on developmental biology and accommodated 700 participants including around 100 non-Japanese scientists.



