Course highlights: "Inorganic Electrochemistry: Fundamentals and Applications in Renewable Energy Catalysis"
Now that the class is almost finished (except for the final exam tomorrow), I'll introduce the highlights of my course "Inorganic Electrochemistry: Fundamentals and Applications in Renewable Energy Catalysis". This is a graduate level course for chemistry students that includes both lectures and laboratory experiments. In the class, we introduced basic principles of electrochemistry, and discusses modern research in the application of transition metal complexes in electrocatalysis for renewable energy storage and production. See the highlights of some of the lab experiments and a syllabus below. Read more...
Here is the syllabus:
- Basic aspects of electrochemistry
- Electrochemical instrumentation
- Cyclic voltammetry: Reversible, irreversible and quasireversible processes
- Cyclic voltammetry: Effect of coupled chemical reactions; Digital simulation of cyclic voltammograms.
- Bulk electrolysis and pulsed voltammetric techniques
- Hydrodynamic techniques: application for studying reaction intermediates and mechanisms.
- Electrochemical behavior of transition metal complexes.
- Redox-active metalloproteins.
- Redox-induced structural reorganization of metal complexes
- Electrocatalysis by transition metals for renewable energy production and storage: water splitting to O2 and H2
- Transition metal-catalyzed electroreduction of CO2 and dehydrogenation of formic acid and alcohols: application for hydrogen storage.
- Immobilization of metal catalysts on electrode surface.
- Photoelectrochemistry
- Application of electrochemical processes in chemical industry.
- Presentations (this year's presentations: "Electrochemistry of Cytochrome P450" and "Nitrogen Fixation")
Below is a brief graphical summary of some experiments that we did over this semester:
Equivalent circuit: modeling CV and electrolysis conditions (see in more detail):
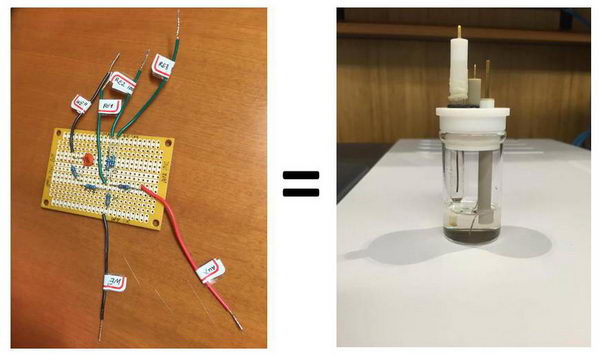
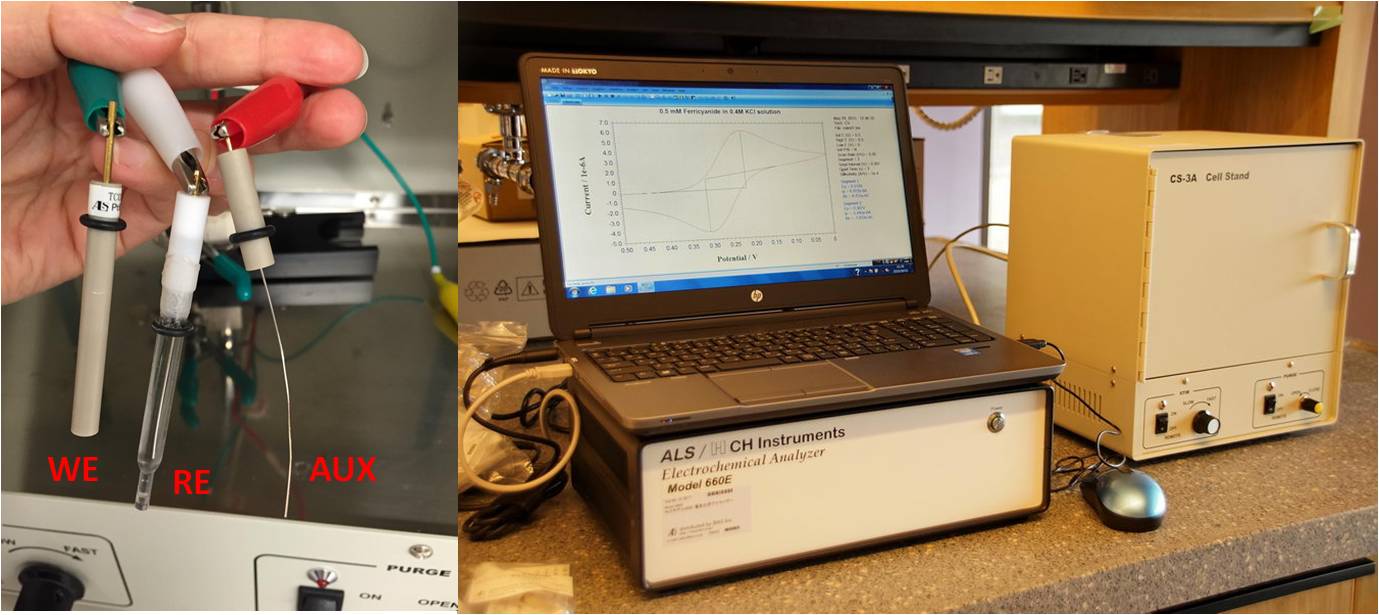
Basics of electrochemistry: instrumentation, electrodes, cells (see relevant post):
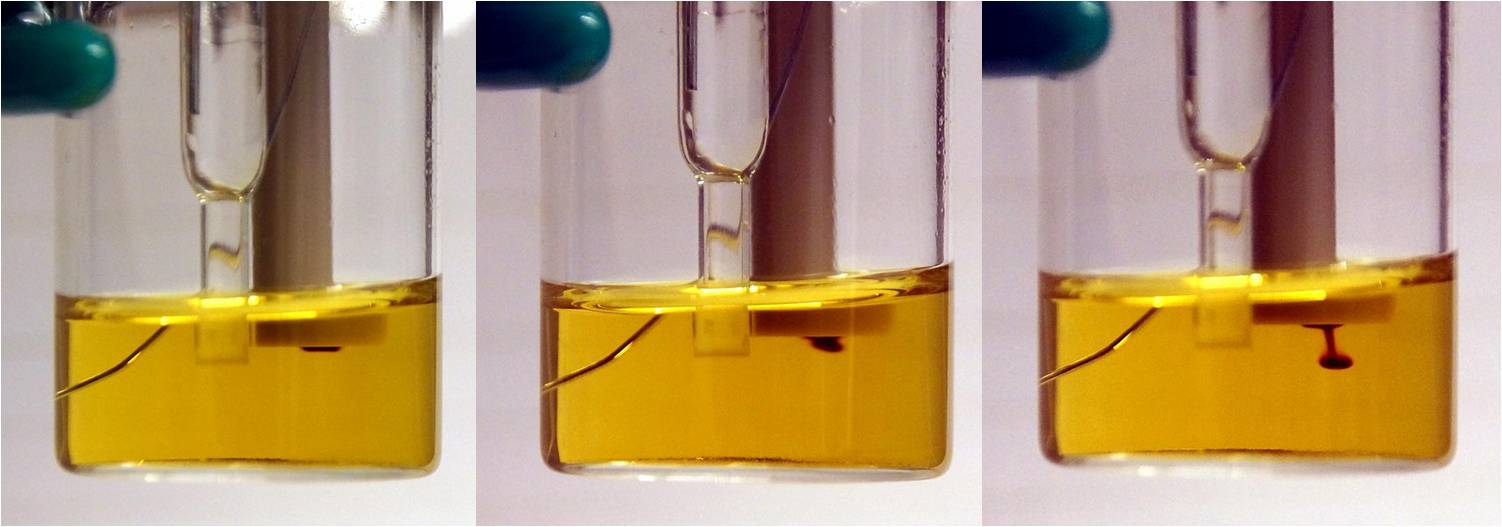
Visualizing mass transport: using a compound that forms oxidized product with a very high extinction coefficient, different mass transport regimes can be visualized. During this process, mass transport at short times when the potential is applied is better described as a linear diffusion, while at longer times it starts transforming to a non-linear, nearly radial diffusion. At long times, convection becomes predominant. Below are the images for different sizes of electrodes (radial is a bit harder to catch and it works better with smaller disk size).

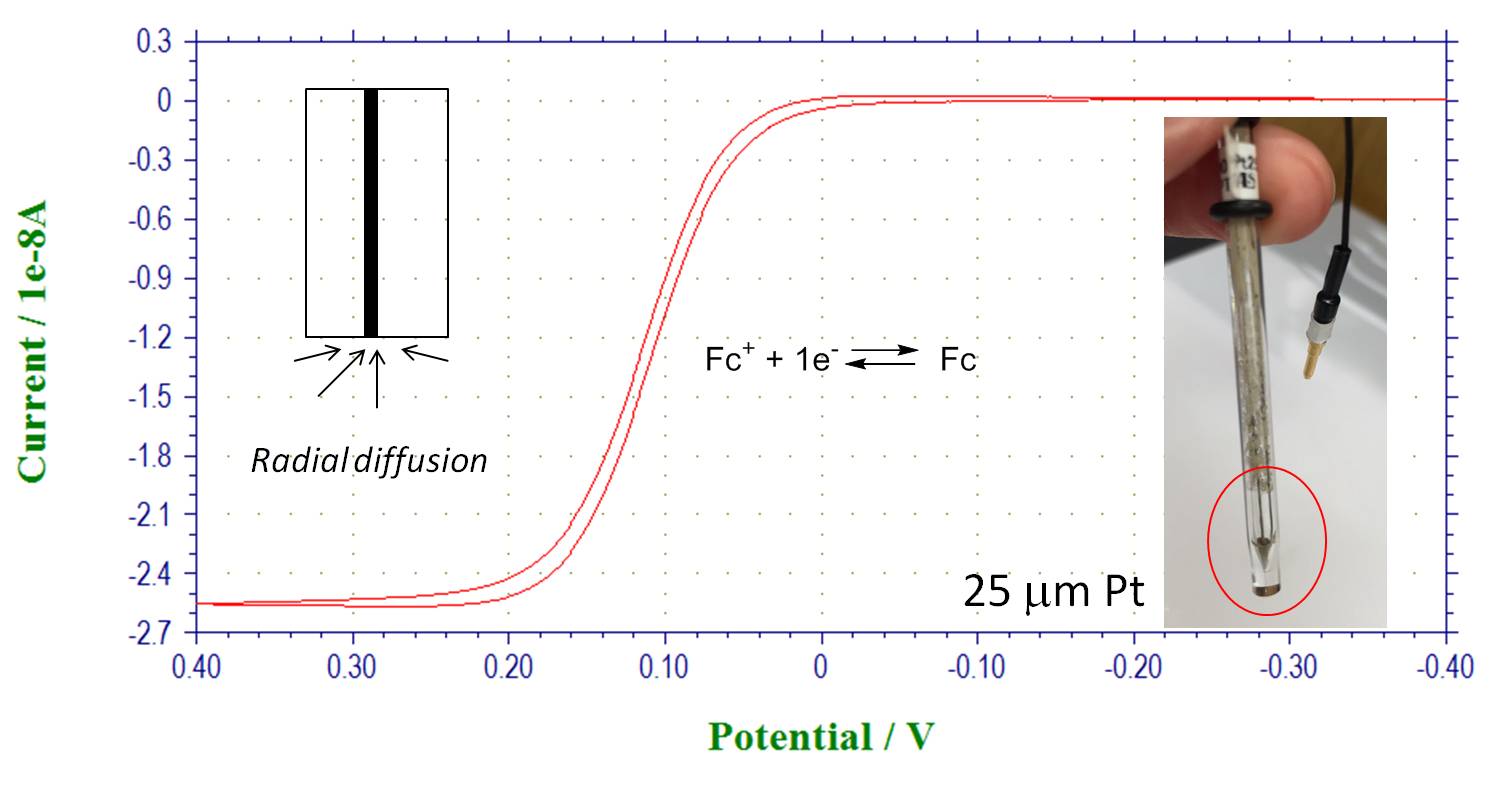
Microelectrodes:
Chemical reactivity coupled with electron transfer. Reduction of chloramphenicol: after reduction scan, a new wave is observed corresponding to electroactive hydroxylamine product.
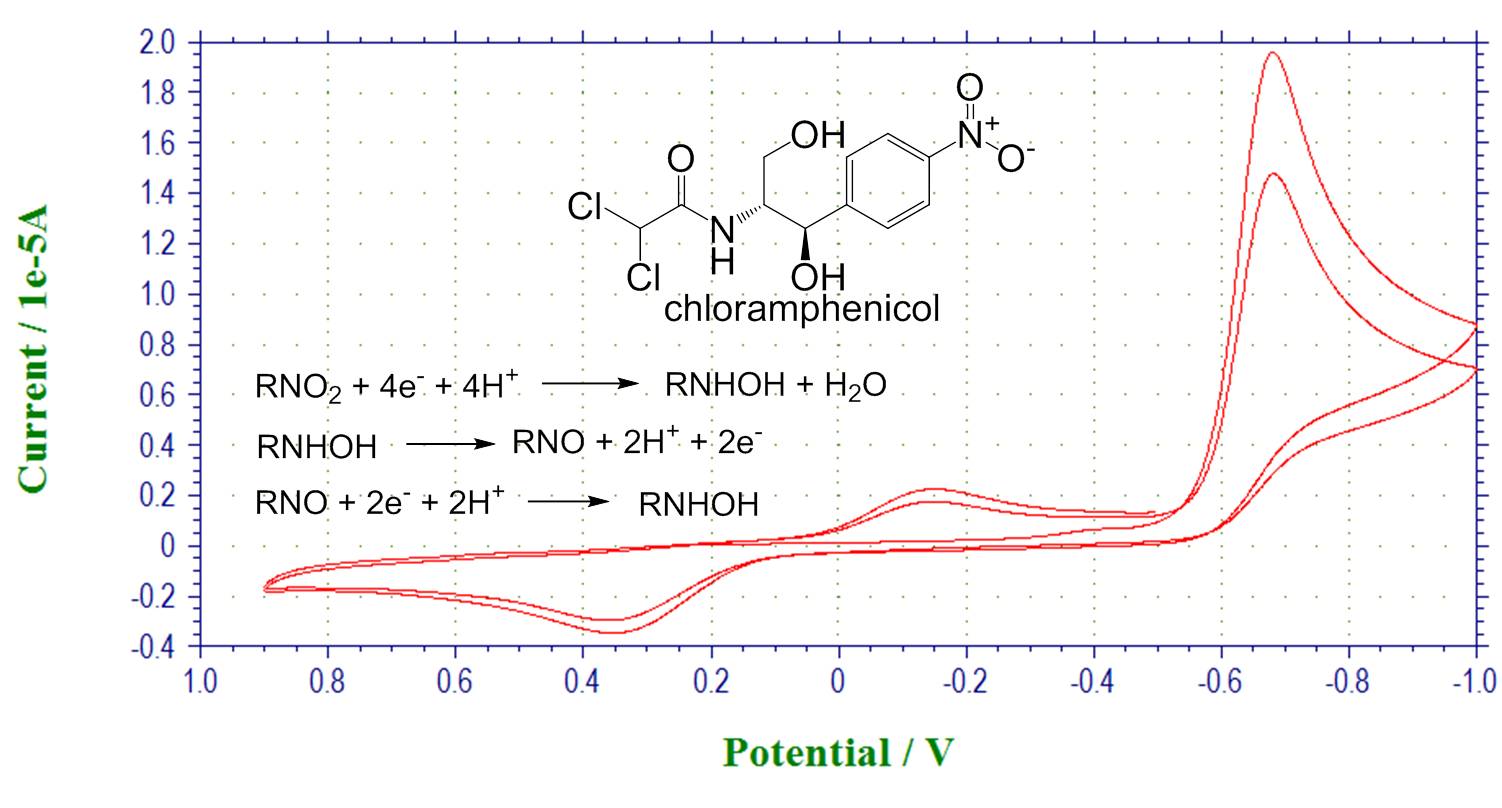
Bulk electrolysis:

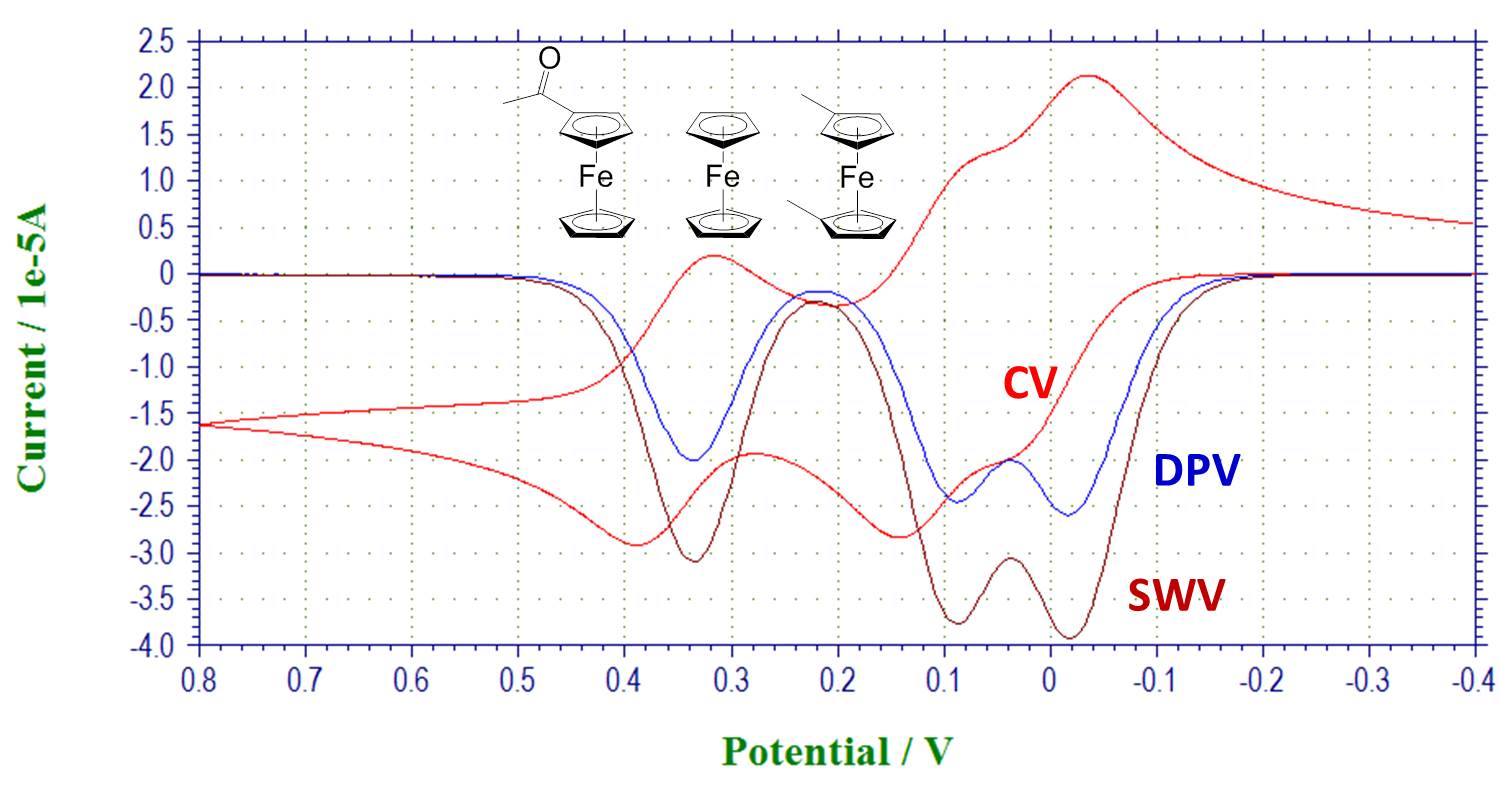
Pulsed techniques to resolve overlapping peaks: overlay shows CV, DPV and SWV of a mixture of substituted ferrocenes:
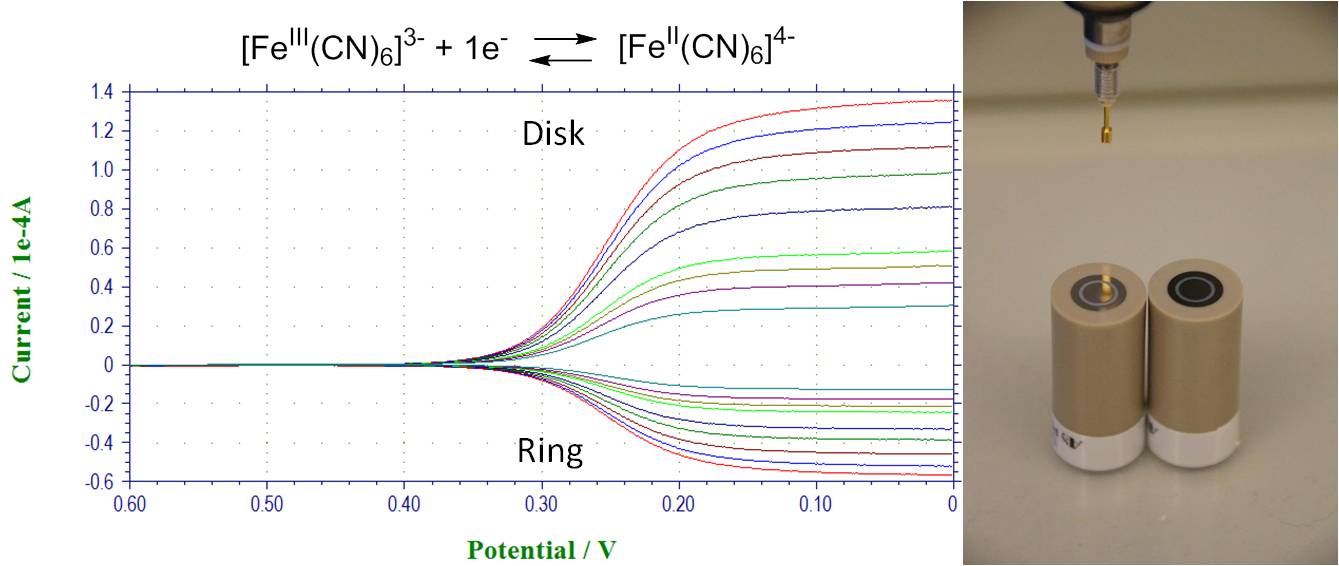
Hydrodynamic methods and their application for dioxygen reduction study. RRDE, RDE:
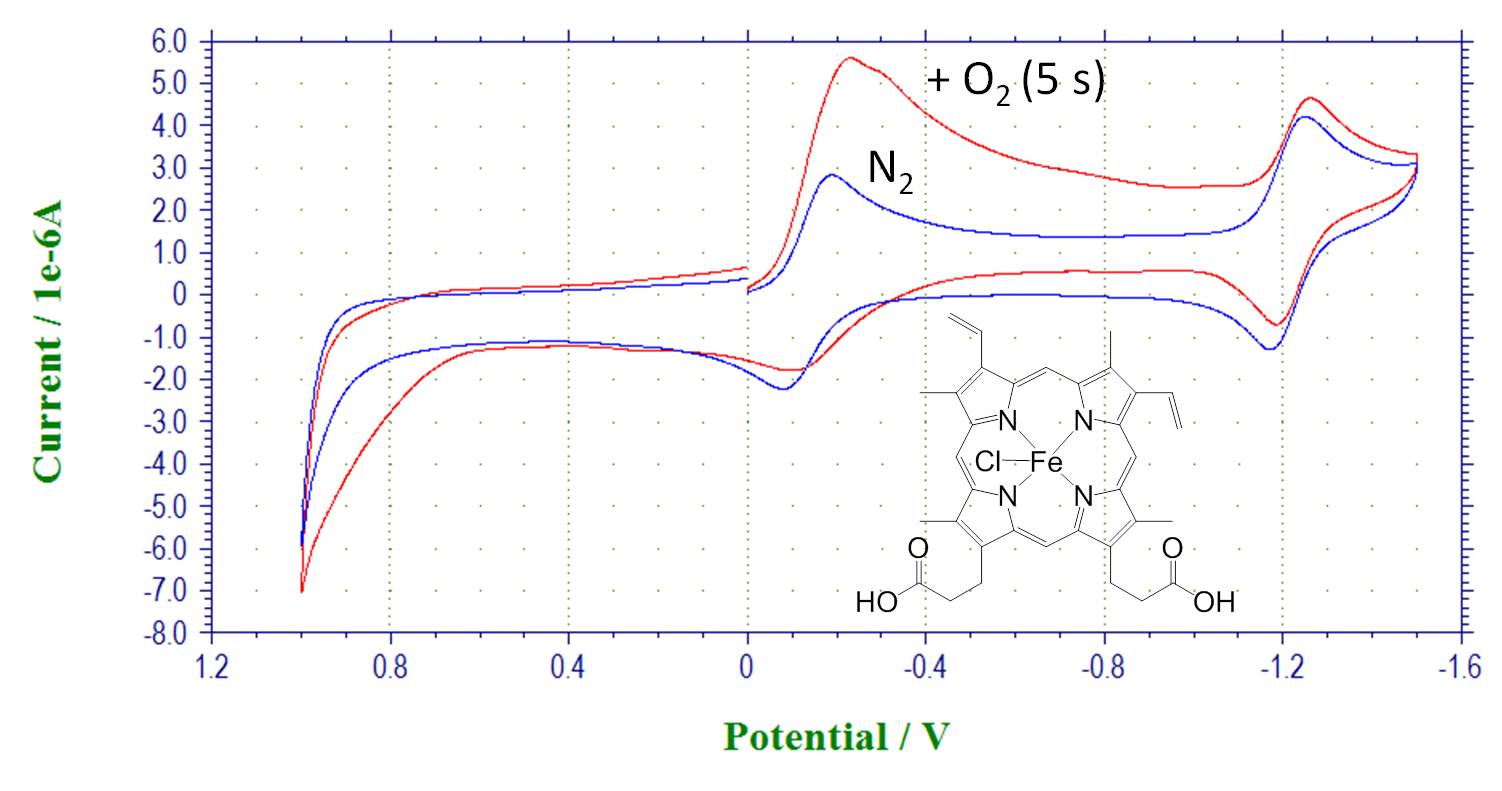
Reactivity of metal complexes with dioxygen and formation of oxygen complex. Hemin, CV in the absence of oxygen and after bubbling oxygen:
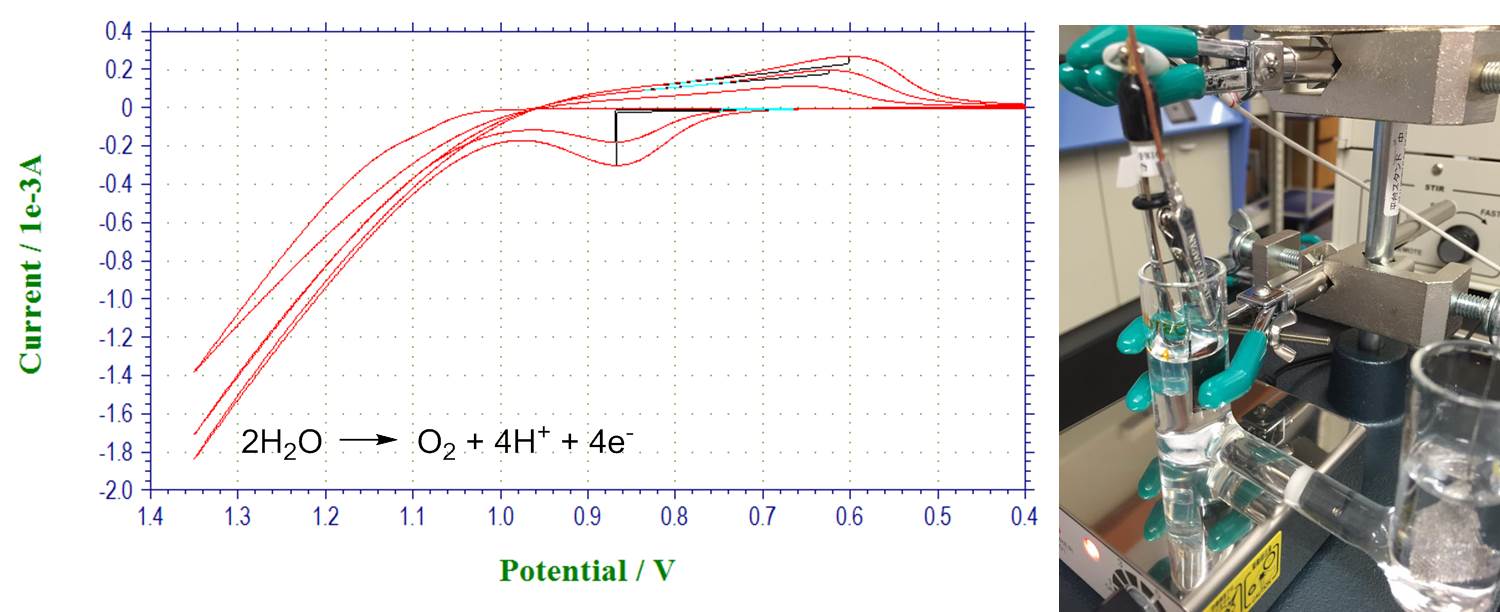
Water oxidation with nickel-borate catalyst: catalyst formation, water electrolysis and Tafel plots (Tafel plots are not shown):
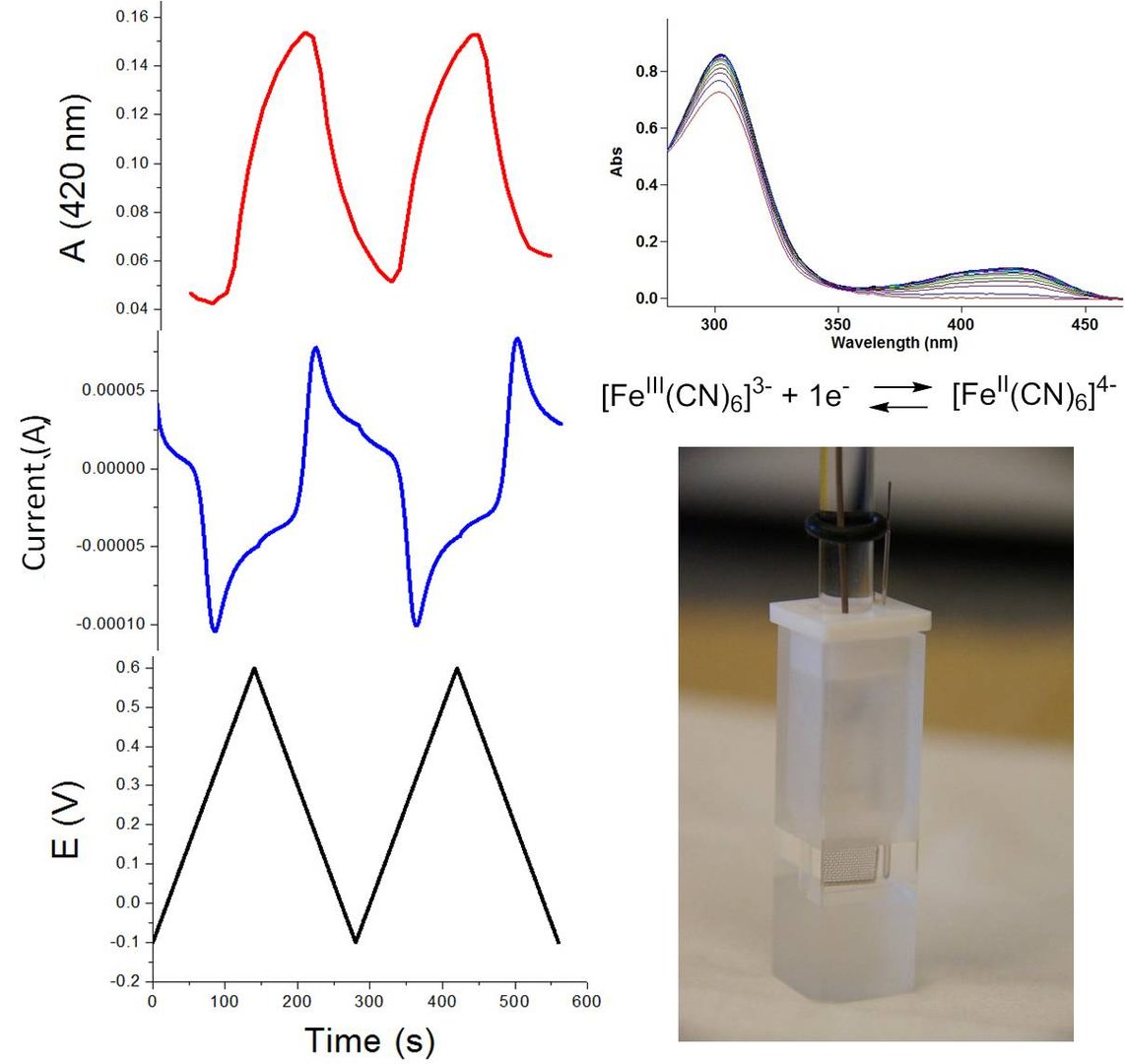
Spectroelectrochemistry: changes in absorbance in the UV-vis spectrum during slow CV scan over several cycles:
Electrogenerated chemiluminescence and chemiluminescence (see more detailed post):


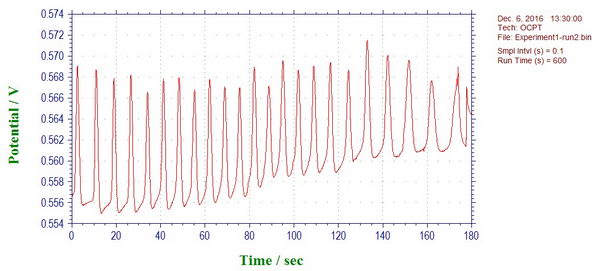
Oscillating reactions and potential (link):
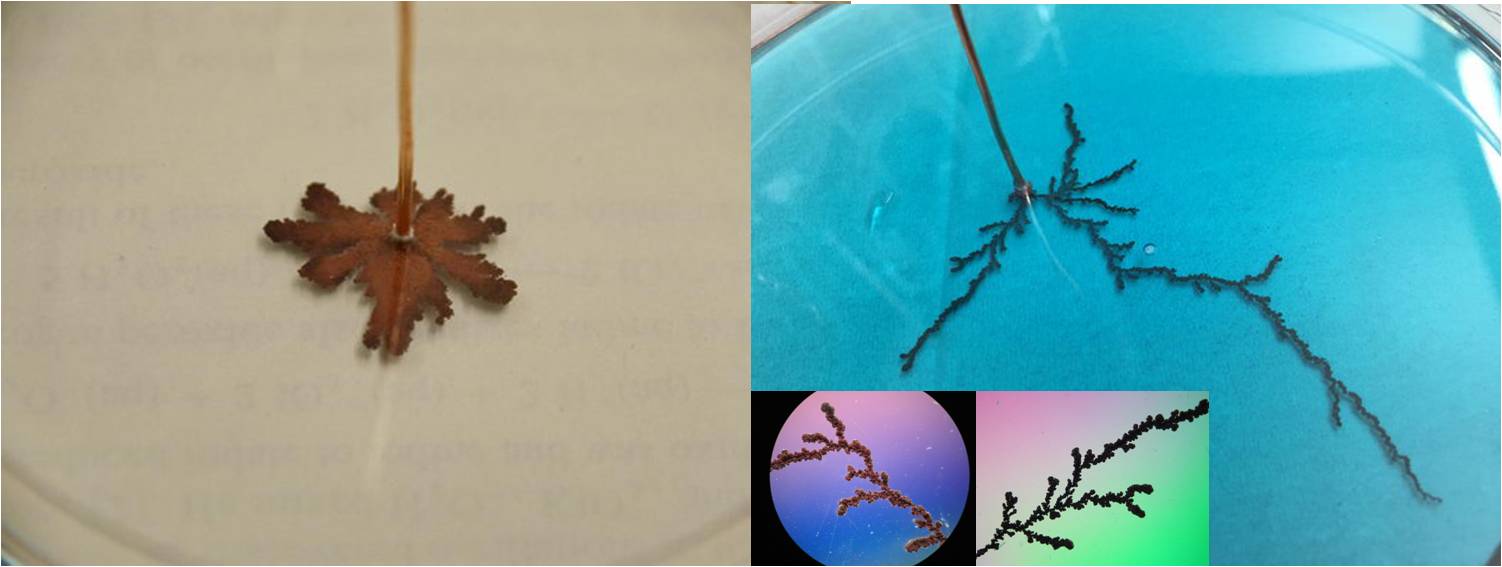
Diffusion-limited aggregation (link):
Electroplating with copper (link):

Many thanks to the TA of the course, Dr. Pradnya Patil, for helping with the experiments:




