FY2021 Annual Report
Cell Signal Unit
Tadashi Yamamoto

Abstract
The Cell Signal Unit studies molecular and cellular events that are relevant to and important for maintaining healthy life. The Unit studies gene regulation that is critical in responding to environmental cues. Particularly, we are interested in CCR4-NOT complex-mediated post-transcriptional regulation in which microRNAs, long non-coding RNAs and RNA binding proteins participates. Unit explores the link between the CCR4-NOT complex and various diseases that include cancer, neuronal disorder, immunological diseases, diabetes/obesity, and developmental failure. The Unit also characterizes protein kinase-mediated cell signaling especially in the control of the brain function such as emotions, learning and memory.
1. Staff
- Dr. Akiko Yanagiya, Staff Scientist
- Dr. Shou Soeda, Postdoctoral Scholar
- Dr. Hiroaki Sako, Postdoctoral Scholar
- Dr. Olga Elisseeva, Visiting Researcher
- Dr. Guillaume Vares, Visiting Researcher
- Dr. Sandrine Anne Laure Burriel, Visiting Researcher
- Dr. Haytham Mohamed Aly Mohamed, Visiting researcher
- Dr. Saori Nishijima, Technical Staff
- Ms. Risa Ishida, Technical Staff
- Ms. Nao Ohmine, Technical Staff
- Ms. Atsuko Sato, Technical Staff
- Mr. Mohieldin Magdy Mahmoud Youssef, Graduate Student
- Ms. Aisulu Maipas, Graduate Student
- Mr. Yuki Tara, Graduate Student
- Mr. Hidenori Onuki, Visiting Research Student
- Ms. Yuki Nakagawa, Research Unit Administrator
- Ms. Chie Narai, Research Unit Administrator
- Ms. Mayu Suzuki, Research Unit Administrator
- Ms. Eriko Okamatsu, Research Unit Administrator
2. Collaborations
2.1 Physiology and Molecular Cellular Biology of the CCR4-NOT complex
- Description: Analyze the physiological and molecular biological roles of each component of the CCR4-NOT complex using gene-modified mice.
- Type of collaboration: Joint research
- Researchers:
- Dr. Keiji Kuba, Department of Physiology, Graduate School of Medicine, Akita University
- Dr. Toru Suzuki, (Division of RNA and Gene Regulation,) The Institute of Medical Science, The University of Tokyo
- Dr. Toshinobu Fujiwara, Laboratory of Biochemistry, Faculty of Pharmacy, Kindai University
- Dr. Taishin Akiyama, RIKEN
- Dr. Masahiro Morita, Department of Molecular Medicine, University of Texas Health Science Center at San Antonio
- Dr. Nahum Sonenberg, Department of Biochemistry, McGill University
2.2 Basic cancer research for prevention and treatment
- Description: Search for substances that contribute to the prevention and treatment of cancer from biological resources, and elucidate its mechanism of action
- Type of collaboration: Joint research I and II
- Researchers:
- I. Representative director Kuniaki Nerome, Nerome Institute of Biological Resources
- II. Professor Shinya Ikematsu, National Institute of Technology, Okinawa College
2.3 Mechanisms and physiological roles of CCR4-NOT complex-regulated gene expression in the brain
- Description: Electrophysiological studies of gene modified mice with abnormal social behavior
- Type of collaboration: Joint research
- Researchers:
- Team leader Masaru Tamura, RIKEN
2.4 Analysis of CNOT9 function during mouse development
- Type of collaboration: Joint research
- Researchers:
- Team leader Hiroshi Hamada, RIKEN
- Technical staff Eriko Kajikawa, RIKEN
2.5 The neuromuscular junction as a new target for treatment of Hereditary Motor and Sensory Neuropathy, Okinawa-type, and related disorders
- Description: Analyzing the pathogenic mechanism of HMSN-P using a mouse model; and analyzing the action mechanism(treatment mechanism) of enhancing NMJK formation on HMSN-P(HMSN with proximal dominancy)-like pathology, at the physiological, and molecular levels.
- Type of collaboration: Joint research
- Researchers:
- Professor Yuji Yamanashi, The University of Tokyo.
3. Activities and Findings
3.1. Neuronal XRN1 is required for maintenance of whole-body metabolic homeostasis
(iScience Volume 24, Issue 10, 22 October 2021, 103151)
Control of mRNA stability and degradation is essential for appropriate gene expression, and its dysregulation causes various disorders, including cancer, neurodegenerative diseases, diabetes, and obesity.
The 5’–3’ exoribonuclease XRN1 executes the last step of RNA decay, but its physiological impact is not well understood. To address this, forebrain-specific Xrn1 conditional knockout mice (Xrn1-cKO) were generated, as Xrn1 null mice were embryonic lethal. Xrn1-cKO mice exhibited obesity with leptin resistance, hyperglycemia, hyper- phagia, and decreased energy expenditure. Obesity resulted from dysregulated communication between the central nervous system and peripheral tissues. Moreover, expression of mRNAs encoding proteins that regulate appetite and energy expenditure was dysregulated in the hypothalamus of Xrn1-cKO mice. Therefore, we propose that XRN1 function in the hypothalamus is critical for maintenance of metabolic homeostasis.
An Image of This Study
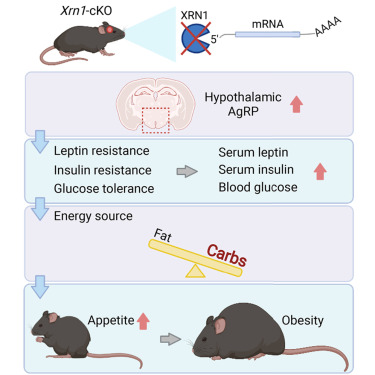
Figure 1: Forebrain specific Xrn1-cKO mice exhibit obesity (left) with hyperphagia (right) at 12 weeks old.
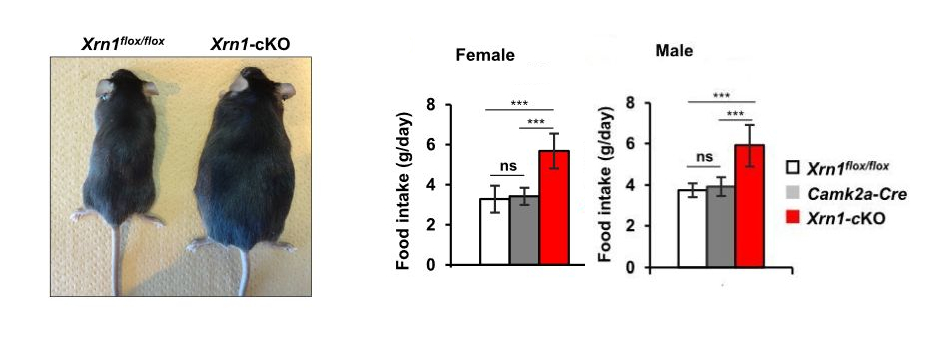
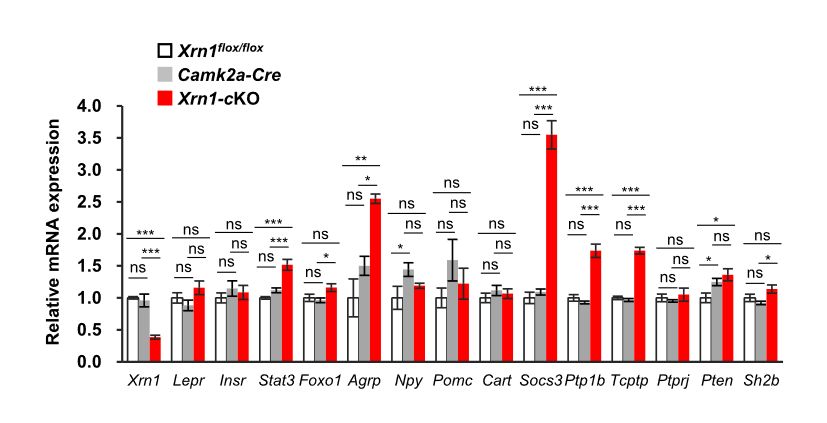
Figure 2: AgRP expression is upregulated in the Xrn1-cKO hypothalamus: Quantitative PCR analysis of the indicated mRNA levels in the hypothalamus of 10 to 14 week-old mice
3.2 Regulation of Early Lymphocyte Development via mRNA Decay Catalyzed by the CCR4-NOT Complex-a review
(Front. Immunol., 19 July 2021)
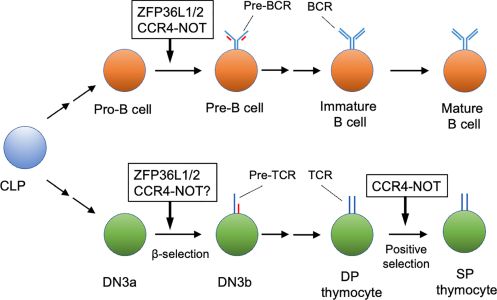
Development of lymphocytes is precisely regulated by various mechanisms. In addition to transcriptional rates, post-transcriptional regulation of mRNA abundance contributes to differentiation of lymphocytes. mRNA decay is a post-transcriptional mechanism controlling mRNA abundance. The carbon catabolite repression 4 (CCR4)-negative on TATA-less (NOT) complex controls mRNA longevity by catalyzing mRNA deadenylation, which is the rate-limiting step in the mRNA decay pathway. mRNA decay, regulated by the CCR4-NOT complex, is required for differentiation of pro-B to pre-B cells and V(D)J recombination in pro-B cells. In this process, it is likely that the RNA-binding proteins, ZFP36 ring finger protein like 1 and 2, recruit the CCR4-NOT complex to specific target mRNAs, thereby inducing cell quiescence of pro-B cells. A recent study showed that the CCR4-NOT complex participates in positive selection of thymocytes. Mechanistically, the CCR4-NOT deadenylase complex inhibits abnormal apoptosis by reducing the expression level of mRNAs encoding pro-apoptotic proteins, which are otherwise up-regulated during positive selection. We discuss mechanisms regulating CCR4-NOT complex-dependent mRNA decay in lymphocyte development and selection.
Figure 1: Active RNA decay pathways mediated by the CCR4-NOT complex. The CCR4-NOT complex can be recruited by RNA binding proteins (RBP), miRNA- Ago2 complex, and YTHDF2 bound to N6-methyladeonsine, which is generated by the METTL3 and METTL14 methyltransferase complexes. The CCR4-NOT complex deadenylates polyA tails of recruited mRNAs. After deadenylation, 5’-decapping enzymes are recruited and eliminate the cap structure. Finally, 5’- exonucleases (Xrn1 and 2) causes degradation of target mRNAs.
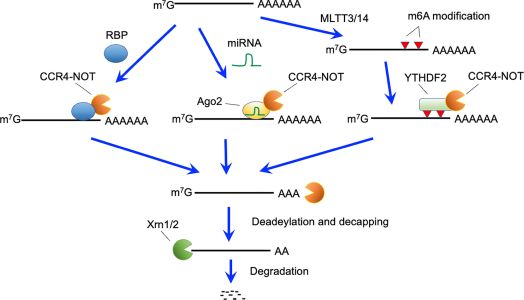
Figure 2: Early lymphocyte development regulated by the CCR4-NOT complex. The CCR4-NOT complex is required for differentiation of pro-B cells to pre-B cells and positive selection of DP thymocytes. ZFP36L1 and ZFP36L2 (ZFP36L1/2) regulate the pro-B cell to pre-B cell transition and b-selection of the DN3 thymocyte stage. Involvement of the CCR4-NOT complex in b selection has not been verified yet. Some differentiation stages were omitted for simplicity. CLP, common lymphoid progenitor; DN, CD4 CD8 double negative thymocyte; DP, CD4 CD8 double positive thymocyte; SP, CD4 or CD8 single positive thymocytes.
4. Publications
4.1 Journals
- Stoney P, Yanagiya A, Nishijima S, Yamamoto T. CNOT7 outcompetes its paralog CNOT8 for integration into the CCR4-NOT complex, Journal of Molecular Biology, 167523, 2022
- Matsuura K, Kobayashi S, Konno K, Yamasaki M, Horiuchi T, Senda T, Hayashi T, Satoh K, Arima-Yoshida F, Iwasaki K, Negishi L, Yasui-Shimizu N, Kohu K, Kawahara K, Kirino Y, Nakamura T, Watanabe M, Yamamoto T, Manabe T, and Akiyama T. SIPA1L1/SPAR1 interacts with the neurabin family of proteins and is involved in GPCR signaling, Journal of Neuroscience, 42 (12) 2448-2473, 2022
- *Suzuki T, Hoshina M, Nishijima S, Hoshina, N, Kikuguchi C, Tomohiro T, Fukao A, Fujiwara T, and *Yamamoto T. Regulation of CCR4-NOT complex deadenylase activity and cellular responses by MK2-dependent phosphorylation of CNOT2, RNA Biology, 19 (1):234-246, 2022
- Akiyama T, Yamamoto T. Regulation of Early Lymphocyte Development via mRNA Decay Catalyzed by the CCR4-NOT Complex, Front Immunol, 12:715675, 2021
- Takaoka S, Yanagiya A, Mohamed H, Higa R, Abe T, Inoue K, Takahashi A, Stoney P and Yamamoto T. Neuronal XRN1 is required for maintenance of whole-body metabolic homeostasis, iScience, 24(10):103151, 2021
- Ban T, Kikuchi M, Sato G, Manabe A, Tagata N, Harita K, Nishiyama A, Nishimura K, Yoshim Ri, Kirino Y, Yanai H, Matsumoto Y, Suzuki S, Hihara H, Ito M, Tsukahara K, Yoshimatsu K, Yamamoto T, Taniguchi T, Nakajima H, Ito S, and Tamura T. Genetic and chemical inhibition of IRF5 suppresses pre-existing mouse lupus-like disease, Nature Communications, 12:4379, 2021
- Minegishi K, Rothé B, Komatsu K, Ono H, Ikawa Y, Nishimura H, Katoh T, Kajikawa E, Sai X, Miyashita E, Takaoka K, Bando K, Kiyonari H, Yamamoto T, Saito H, Constam D, and Hamada H. Fluid flow-induced left-right asymmetric decay of Dand5 mRNA in the mouse embryo requires a Bicc1-Ccr4 RNA degradation complex, Nature Communications, 12:4071, 2021
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Y. Tara, A. Yanagiya, D. Mostafa, T. Yamamoto, mRNA turnover mediated by RNA modification to maintain pancreatic β cell homeostasis, RNA frontier meeting, Okinawa, Japan, March 3, 2022, online
- H. Sako, M. Youssef, O. Elisseeva, T. Akimoto, K. Suzuki, T. Ushida, T. Yamamoto, microRNAs slow translating ribosomes to prevent protein misfolding, RNA Frontier meeting, Okinawa, Japan, March 3, 2022, online
- Y. Tara, A. Yanagiya, D. Mostafa, T. Yamamoto, The postr-transcriptional regulation by the CCR4-NOT complex is essential for pancreatic β cell function, The 8th CCR4-NOT Meeting, Ito, Japan, December 6, 2021, onsite
- S. Nishijima, T. Suzuki, T. Yamamoto, Possible involvement of CNOT11, a subunit of CCR4-NOT complex, in the control of programmed cell death, The 8th CCR4-NOT Meeting, Ito, Japan, December 6, 2021, onsite
- L. Picard, T. Yamamoto, Exploring the interplay between m6A methylation and CCR4-NOT during Influenza infection, The 8th CCR4-NOT Meeting, Ito, Japan, December 6, 2021, onsite
- H. Sako, M. Youssef, O. Elisseeva, T. Akimoto, K. Suzuki, T. Ushida, T. Yamamoto, miRNAs slow translating ribosomes to prevent protein misfolding in a CCR4-NOT-dependent manner, The 8th CCR4-NOT Meeting, Ito, Japan, December 5, 2021, onsite
- T. Yamamoto, Physiology of CCR4-NOT learned from KO mice study, The 8th CCR4-NOT Meeting, Ito, Japan, December 5, 2021, onsite
- M. Tokumasu, A. Sato, T. Kureha, T. Yamamoto, 乳癌細胞におけるTobの発現とNF-κBシグナルの制御, The 44th Annual Meeting of the Molecular Biology Society of Japan, Yokohama, Japan, December 3, 2021, online
- S. Nishijima, T. Suzuki, T. Yamamoto, Possible involvement of CNOT11, a subunit of CCR4-NOT complex, in the control of programmed cell death, The 44th Annual Meeting of the Molecular Biology Society of Japan, Yokohama, Japan, December 3, 2021, online
- A. Maipas, P. Stoney, T. Yamamoto, The contribution of post-transcriptional regulation to cellular senescence, The 44th Annual Meeting of the Molecular Biology Society of Japan, Yokohama, Japan, December 2, 2021, onsite
- N. Ohmine, S. Ikematsu, T. Yamamoto, Molecular mechanism of Oxyresveratrol-mediated growth inhibition in cancer cells, The 44th the Molecular Biology Society of Japan, Yokohama, Japan, December 2, 2021, online
- S. Soeda, T. Yamamoto, マウス卵細胞におけるCCR4-NOT複合体によるmRNA poly-A鎖長制御の生理的意義, The 44th Annual Meeting of the Molecular Biology Society of Japan, Yokohama, Japan, December 2, 2021, onsite
- A. Yanagiya, Y. Tara, T. Yamamoto, Translational control is a potential therapeutic target to cure a variety of diseases: Translational control of m6A-methylated mRNAs to maintain pancreatic β cell homeostasis, The 44th the Molecular Biology Society of Japan, Yokohama, Japan, December 1, 2021, onsite
- A. Yanagiya, T. Yamamoto, 膵β細胞の恒常性維持に重要なCCR4-NOT複合体を介したmRNA分解制御の解析, The 11th Signaling network meeting, Osaka, Japan, November 26, 2021, online
- T. Yamamoto, The OIST concept, Memorial Ceremony Prof. Teruko Tamura-Neiman, The Future of interdiciplinary and internaional science and support to young scientists, Hannover, Germany, September 27, 2021, onsite
- M. Youssef, Y. Kiyama, H. Hamada, E. Lai, M. El-Tabbal, T. Yamamoto, Role of Transducer of ErbB2 (Tob) protein in the brain through regulating stress-induced behavior, The 44th Annual Meeting of the Japan Neuroscience Society, Kobe, Japan, July 28, 2021, onsite
- A. Maipas, P. Stoney, T. Yamamoto, The contribution of post-transcriptional regulation to cellular senescence, The 26th Annual Meeting of the RNA Society, Okinawa, Japan, May 29, 2021, online, (Poster Award)
- T. Yamamoto, CCR4-NOT複合体による転写後制御:原腸胚形成におけるCNOT9の役割, 微研講演会, Suita, Japan, April 27, 2021, online
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 Seminars
6.1.1. "When designing dream vaccines, consider the memory B cell behavior"
- Date: April 2, 2021
- Venue: OIST Campus Lab4
- Speaker: Prof. Tomohiro Kurosaki, Osaka University
6.1.2. "Novel function of wild-type p53 in cancer cells"
- Date: April 7, 2021
- Venue: OIST Campus Center Bldg.
- Speaker: Dr. Rieko Ohki, National Cancer Center Research Institute
6.1.3. "Phase separation and a novel anti-cancer compound ACA-28 as regulators of PKC/MAPKsignaling dynamics"
- Date: April 14, 2021
- Venue: OIST Campus Lab4
- Speaker: Prof. Reiko Sugiura, Kindai University
6.1.4. "Clock in clocks: how clock ticking in the heart ofthe cell is coupled to diurnal rhythms"
- Date: November 19, 2021
- Venue: OIST Campus Center Bldg.
- Speaker: Prof. Hitoshi Okamura, Kyoto University
6.1.5. "Systemic host response to virus and thedevelopment of precision medicine for infectiousdisease"
- Date: November 22, 2021
- Venue: OIST Campus Center Bldg.
- Speaker: Dr. Yumiko Imai, National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN)
6.1.6. "Orchestrating inter-organ communication and treating metabolic disorders by mRNA decay of hepatokines"
- Date: December 20, 2021
- Venue: OIST Campus Center Bldg.
- Speaker: Dr. Sakie Katsumura, University of Texas Health Science Center at San Antonio
6.1.7. "EHE – Epithelial Hemangio-Endothelioma, cancer driven by fusions of YAP or TAZ oncogenes with transcription factors"
- Date: January 19, 2022
- Venue: Online
- Speaker: Prof. Marius Sudol, Icahn School of Medicine at Mount Sinai
6.1.8. "How Aurora B activity is enriched at centromeres in mitosis"
- Date: January 26, 2022
- Venue: Online
- Speaker: Dr. Toru Hirota, The Cancer Institute of the Japanese Foundation for Cancer Research (JFCR)
6.1.9. "The impact of deep learning on cancer research"
- Date: February 2, 2022
- Venue: Online
- Speaker: Prof. Tatsuhiko Tsunoda, The University of Tokyo
6.1.10. "“Humanized Mice” for understanding and targeting poor prognosis human leukemia"
- Date: February 16, 2022
- Venue: Online
- Speaker: Dr. Fumihiko Ishikawa, RIKEN Integrative Medical Sciences
6.1.11. "Novel perspective of the usage of ROS inducers for cancer therapy"
- Date: March 2, 2022
- Venue: OIST Campus Lab1
- Speaker: Dr. Nobumoto Watanabe, RIKEN Center for Sustainable Resource Science (CSRS)
6.1.12. "Mechanism of inducing T cell senescence followed by inflammation diseases"
- Date: March 9, 2022
- Venue: OIST Campus Lab1
- Speaker: Dr. Takashi Saito, RIKEN Center for Integrative Medical Sciences
6.1.13. "Identification of a novel cell surface SARS-CoV-2 entry pathway, which is metalloproteinase-dependent but TMPRSS2-independnt"
- Date: March 16, 2022
- Venue: OIST Campus Lab1
- Speaker: Prof. Jun-ichiro Inoue, The University of Tokyo
6.1.14. "Metastasis of Breast Cancer – Studies using organoriented metastatic cell lines"
- Date: March 23, 2022
- Venue: Online
- Speaker: Prof. Kentaro Semba, Waseda University
6.1.15. "A novel cancer therapy to stimulate oncogenic ERK signaling in melanoma and pancreatic cancer cells"
- Date: March 30, 2022
- Venue: OIST Campus Lab1
- Speaker: Prof. Reiko Sugiura, Kindai University
6.2 Meetings
Nothing to report
7. Other
Nothing to report.



