FY2022 Annual Report
Marine Climate Change Unit
Professor Timothy Ravasi

Chengze Li, Jeffrey Jolly, Jamila Rodrigues, Michael Izumiyama, Billy Moore, Ayşe Haruka Oshima Açıkbaş, Timothy Ravasi, Alexandru Mihai, Roger Huerlimann, Taewoo Ryu
Hsiao-chian Chen, Yoko Shintani, Erina Kawai, Callum James Hudson, Nicolas Dierckxsens, Kaisar Dauyey, Shannon McMahon
Abstract
Our unit aims to understand how coral reef fish adapt to environmental changes such as climate change, heatwaves, overfishing and urbanization. Earth's oceans are warming and acidifying due to increasing anthropogenic CO2 production, and extreme events such as marine heatwaves are increasing in frequency, duration, and magnitude. Coral reef fish are especially vulnerable to spikes in temperature because they are already adapted to living close to their thermal limits. Determining which species can adapt to rapid environmental change, and how they will do so, is critical for predicting the ecological effects of climate change and their impact on the world economy.
In our laboratory research, we investigate how short-term as well as heritable changes in gene expression in multiple fish species are affected by increases in sea level temperature and acidity. We study animals that are descended from wild breeding pairs and have been reared in our aquariums over several generations. Using a unique device called the Heatwaves Simulator aquarium system based at OIST’s Marine Science Station, we expose fish to specific conditions, including the temperature and acidity level predicted to occur in seawater by the end of the century. We combine these manipulations with a range of advanced genomic technologies to identify the cellular mechanisms that allow coral reef fish communities in Okinawa and around the world to acclimate and adapt to changing conditions.
1. Staff
- Tae Woo Ryu, Research Unit Group Leader
- Roger Huerlimann, Postdoctoral Scholar
- Shannon McMahon, Postdoctoral Scholar
- Nicolas Dierckxsens, OIST Interdisciplinary Postdoctoral Scholar (50/50 with Nick Luscombe Unit)
- Erina Kawai, Research Unit Scientific Diver
- Jeffrey Jolly, Research Unit Technician
- Chengze Li, Research Unit Technician
- Gelyn Bourguignon, Fish Husbandry Technician
- Michael Izumiyama, Graduate Student
- Billy Moore, Graduate Student
- Callum Hudson, Graduate Student
- Ayşe Haruka Oshima Açıkbaş, Graduate Student
- Kaisar Dauyey, Special Research Student
- Johanna Linnea Johansson, Research Intern
- Yoko Shintani, Research Unit Administrator
2. Collaborations
2.1 Anemonefish development and genomics
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor Vincent Laudet, OIST, Japan
2.2 Coral Reef fish adaptation to marine heatwaves
- Type of collaboration: Scientific Collaboration
- Researchers:
- Professor Philip Munday, James Cook University, Australia
- Professor Jennifer Donelson, James Cook University, Australia
- Professor Jacob Johansen, University of Hawaii
2.3 Coral Reef fish adaptation to Ocean Acidification
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor Celia Schunter, The University of Hong Kong, HongKong
2.4 Coral Reef fish genomics
- Type of collaboration: Scientific Collaboration
- Researchers:
- Deputy Director Piero Carninci, RIKEN Center for Integrative Medical Sciences, Japan
- Professor Jesper Tegnér, KAUST, Saudi Arabia
- Professor Valerio Orlando, KAUST, Saudi Arabia
2.5 Coral Reef fish Physiology
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor Martin Grossel, University of Miami, USA
2.6 Impact of coastal development on coral reef ecosystems
- Type of collaboration: Scientific Collaboration
- Researchers:
- Professor James Davis Reimer, University of Ryukyus, Japan
- Professor Noriyuki Satoh, OIST, Japan
2.7 Natural analogues of climate stressors
- Type of collaboration: Scientific Collaboration
- Researcher:
- Dr. Riccardo Rodolfo-Metalpha, UMR ENTROPIE, Nouvelle Calédonie
2.8 Natural Volcanic CO2 Analogues
- Type of collaboration: Scientific Collaboration
- Researchers:
- Professor Ivan Nagelkerken, The University of Adelaide, Australia
- Professor Sylvain Agostini, University of Tsukuba, Japan
2.9 Okinawa Coral spawning and development
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor David J. Miller, James Cook University, Australia
2.10 Sharks physiology under climate stressors
- Type of collaboration: Scientific Collaboration
- Researchers:
- Professor Jodie L. Rummer, James Cook University, Australia
- Professor Rui Rosa, Faculdade de Ciências da Universidade de Lisboa, Portugal
2.11 Transgenerational acclimation
- Type of collaboration: Scientific Collaboration
- Researcher:
- Professor Jennifer Donelson, James Cook University, Australia
3. Activities and Findings
3.1 Design and Construction of the OIST Heatwaves Simulator aquariums system.
This year our unit in collaboration with Luxaqua, we designed and constructed the OIST Heatwaves Simulator, a unique aquariums system that will allows us and our collaborators to recreate a future environment including the simulation of marine heatwaves at the OIST Marine Science Station.
The OIST Heatwaves Simulator consist of three separate tank systems (Figure 1); 1) 10, 100L outdoor breeding pair tanks that will be used to house brood stock breeding pairs, 2) 10, 75L larval rearing tanks, that from 2022 onwards (F2, F3) will be used in the larval rearing stage of this multi-generational experiment, 3) 144, 55L tanks that will house experimental juvenile fish in different treatments. Temperature within these experimental tanks is controlled by four inline heaters, that allow five different temperature treatments to be specified in each individual tank. Temperature is controlled and monitored by automated software and associated temperature probes. Software also has remote monitoring and control capabilities.
This system is unique in the world and it will help to incentive collaborators from Japan and overseas to come to OIST and work with us at the marine station.

Figure 1. The OIST Heatwaves Simulator
3.2 Coral Reef fish Physiology and Metabolic Performance
To assess the effects of the different heatwave treatments on coral reef fish’s metabolic rates, in collaboration with Loligo Systems, we set up at the OIST Marine Station a 170ml mini-swim tunnel (Figure 1). Water flow within the swim tunnel is generated by a propeller, and laminar flow is created by a series of flow straightening plastic honeycomb meshes. The velocity of water within the tunnel is controlled by Loligo Systems AutoResp software and is calibrated using Loligo Systems fluorescent PE microspheres and the associated Digital Particle Tracking Velocity software. An oxygen dipping probe is inserted into the swim tunnel, allowing for continuous measurements of oxygen concentration within the tunnel. A pump can be used to flush the swim tunnel with fresh seawater from the waterbath if required. The swim tunnel is submerged within a temperature controlled waterbath allowing the temperature within the swim tunnel to be manipulated. Flushing of the swim-tunnel and resting chambers, monitoring of oxygen concentrations and temperature, and calculations of oxygen consumption rates are all completed automatically by Loligo Systems AutoResp software.
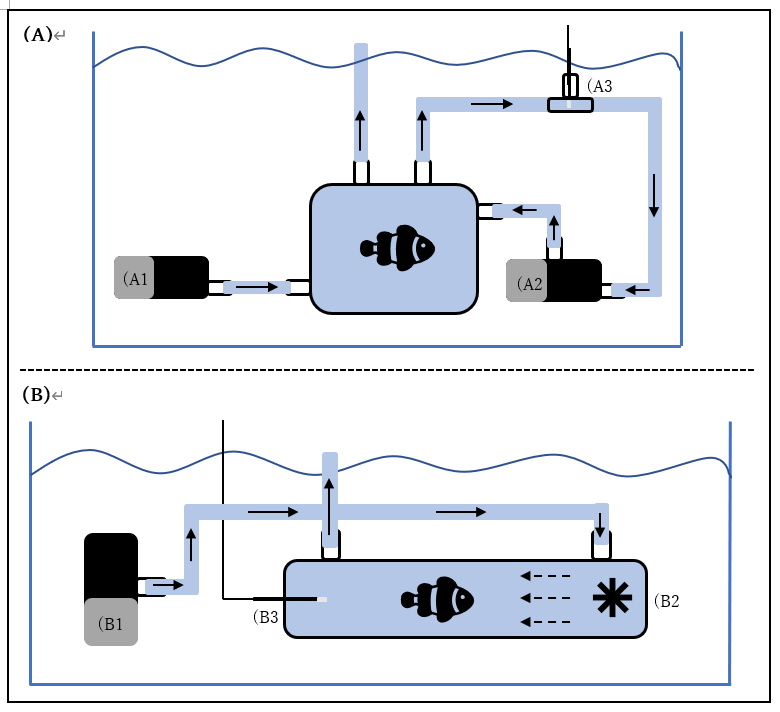
Figure 2. (A) Schematic of one Loligo Systems resting chamber set-up used to quantify RMR. A pump (A1) is used to flush the chamber with fresh seawater from the waterbath. A pump (A2) is used to recirculate water through a closed loop that passes through a flow through oxygen cell, within which oxygen concentration is measured by a fibre optic oxygen sensor (A3). (B) Schematic of a Loligo Systems 170ml mini-swim tunnel used to quantify MMR. A pump (B1) is used to flush the swim tunnel with fresh seawater from the waterbath. Water flow is generated within the swim tunnel by a propeller (B2) and oxygen concentration is measured by a 200mm oxygen dipping probe inserted into the opposite end of the tunnel (B3).
3.3 Amphiprion ocellaris Spawning and Larval Rearing
In order to conduct the planned long-term multi-generational experiment detailed above, we first needed to ensure that wild-caught A.ocellaris from Okinawan coral reef systems would spawn within aquaria. To test this, one breeding pair of A.ocellaris was acquired from local fishermen in September 2019. This pair was placed within a 200L aquaria at OIST Marine Science Station with a sea anemone (Heteractis magnifica) and multiple rocks.

Figure 3. Images displaying (a) fertilized eggs of A.ocellaris (b) larval rearing tank and (c) juvenile A.ocellaris 1 month after hatching within aquaria at OIST marine science station.
On the 31st of May 2020 eggs were observed within the breeding pair tank (Figure 1). The development of eggs was tracked using visual observation and comparisons to know stages of embryonic development. On the 7th of June, eggs were transferred to a larval rearing tank for hatching (Figure 3) and larval A.ocellaris were present in the tank on the 8th of June.
The successful spawning and larval rearing program of 2020 allowed us to develop for the first time at OIST Marine Science Station a larval rearing protocol.
3.4 The Sequencing of Amphiprion ocellaris, Amphiprion clarkii and Epinephelus malabaricus genomes.
Our unit sequenced the genomes of three coral reef fish from the water of Okinawa island, the Amphiprion ocellaris, Amphiprion clarkii and Epinephelus malabaricus (Table 1). All these genomes have been sequenced using PacBio long-reads sequencing combined with Illumina gDNA and transcriptome sequencing and binned into chromosomes using Hi-Seq contact map technology.
These genomes are among the most completed fish genomes ever sequenced and are available free for the entire community.
Figure 4. The phylogeny of Anemonefish
Figure 5. The Chromosome-scale genome of the Clark's anemonefish (Amphiprion clarkii)
3.5 Natural Analogues of Future Climate
Unique sites which are natural analogues of future ocean have a community that already exists under this naturally occurring extreme environment and can be utilized as a natural laboratory. These sites are subjected to daily changes in ocean chemistry, have dynamic interactions between habitat, predator, and prey, and have existed in long timescales. Inhabitants may have also adapted to these extreme conditions through generations. Although conditions at each extreme site vary and are not perfect simulations of future oceans, they can provide a window into how species may respond to climate change.
Our unit successful performed field expeditions at the following natural analogues.
Bourake, New Caledonia
In the spring of 2020, with the collaboration of the IRD and several other universities, we conducted a two-week expedition to Bourake, New Caledonia, where we collected four species of fishes (Dascyllus aruanus, Zoramia leptacantha, Chaetodon lunulatus, Pomacentrus molluscensis) from future and control sites.
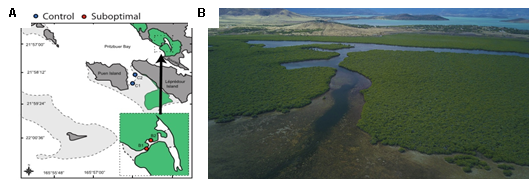


Figure 6. Map of Bourake, New Caledonia. A. The red dots represent the lagoon (future condition) where hot deoxygenated water flows during the outgoing tide. Blue dots represent control reefs (current condition) B. Photos of the lagoon.
Ioutorijima, Japan
In the fall of 2020, our team and collaborators from Tokyo University and the University of the Ryukyus conducted a three-day trip to the uninhabited island Ioutorijima. We collected eDNA samples and placed baited remote underwater cameras (BRUV) at the CO2 seep. We collected eDNA samples from the seep (future) and a nearby reef (control). We placed BRUVs with krill to determine the species that were present at the seep. Additionally, this trip produced a publication led by Reimer et al. (2021), which discovered and described a rare occurrence of aragonite-forming Nanipora at the CO2 seep site.
Figure 7. Map of Ioutorijima, Okinawa. The red circle indicated the CO2 Seep site, and the blue circle indicates the control reef. Water samples were collected at the yellow marker at the control site and the star located at the seep site.


Figure 8. Underwater photos of the habitat at CO2 Seep site (left) and Control reef (right). The bubbles are CO2 seeping from the ground.
Shikine island, Japan
In December 2020, our team conducted preliminary work for our project at Shikine island, Japan. Water samples for eDNA analysis were collected from the seep and control site. We also conducted a visual survey of the seep and control site to determine the species we will collect for our future study. The seep site was dominated by turf algae and had many herbivores similar to Ioutorijima.
The third image, Figure 3, is a single image with the caption below it.
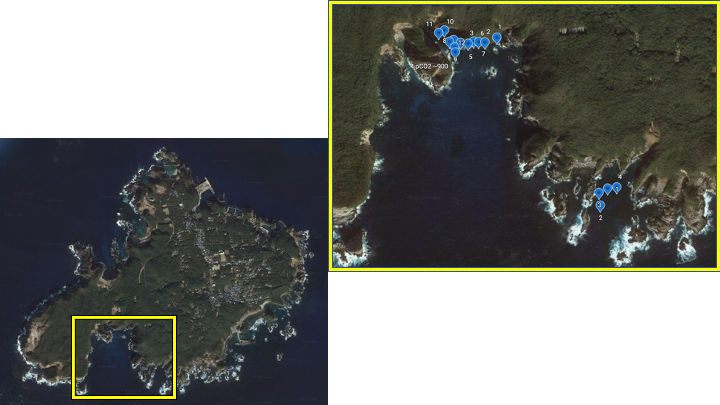
Figure 9. Map of Shikine island and an expanded view of both control and seep site. The seep site is located at the top, and the control reef is at the bottom of the map. The blue dots represent where we collected water samples for eDNA analysis.


Figure 10. Underwater image of CO2 seep (left) control reef (right) at Shikine island. The CO2 seep site was covered in turf algae compared to the control reef, dominated by coral.
3.6 Tropicalizing Range-Shifts in the Japanese Coastlines via Environmental DNA Metabarcoding (eDNA).
Since the industrial age, anthropogenic activities have resulted in a consistent increase of sea surface temperature and ocean acidification, causing a world-wide shuffling of species, as part of an overall poleward range shift in both terrestrial and aquatic ecosystems. Specifically for coral reef recruitment, a tropical decrease and subtropical increase at the 20° latitude have been recorded with shifts to the more temperate areas of Southern Japan. This “tropicalization” raises the questions whether the Ryukyu arc islands could be experiencing this poleward shift along its axis (taking into account the effect of the Kuroshio current) and if it is also acting as a refugia for the migrating coral reef communities from near the equator.
Are the high latitude coral communities in Okinawa and Southern Japan the future areas of refuge for the range-shifting reef organisms, with the help of Kuroshio? Even if so, how would all the coastal development taking place in Okinawa affect this in the short-term and how immediately disruptive it could be? Could these migrating organisms be fitter compared to the original residents and what implications does this have for the future biodiversity of Okinawa? And how could the climate change be affecting the speciation process in the long-term, when compared to the past drastic changing phases in the climate?
The current climate-driven range shifts and therefore the opening-up of novel niches could serve as a window to see into and characterize the process of speciation with hybridization and adaptive radiation. Searching for and studying these newly created potential hybrid zones, we could gain a better understanding of speciation mechanisms in a changing and connected ecosystem. This hybrid swarm theory of adaptive radiation could be tested using population genetics and transcriptomics, in the nature and in the laboratory using the clownfish model organism.
In an increasingly warm world, it is crucial to understand how the changing conditions are affecting the species for more effective mitigation and conservation efforts. Effective design and management of MPAs can be achieved using the information gained from population connectivity and genomics studies, which can point to important sites of refuge/recruitment/nurseries. Temporal and spatial sampling for and studying eDNA across Okinawa and Japan can characterize the effects of the immediately disruptive anthropogenic activities such as coastal development and reconfiguration on biodiversity.

Figure 11. Marine Climate Change Unit members collecting and filtering seawater for environmental DNA (eDNA) studies in several location across Japan.
4. Publications
4.1 Journals
Book
- Evolution, development and ecology of anemonefishes: model organisms for marine science. V Laudet and T Ravasi. CRC Press, 2022. https://www.amazon.co.jp/-/en/dp/0367645815.
Journal articles
- Research priorities for the sustainability of coral-rich western Pacific seascapes. GS Cumming, M Adamska, ML Barnes, J Barnett, D Bellwood, J Cinner, P Cohen, J Donelson, K Fabricius, RQ Grafton, A Grech, GG Gurney, O Hoegh-Guldberg, AS Hoey, MO Hoogenboom, J Lau, C Lovelock, R Lowe, D Miller, TH Morrison, PJ Mumby, M Nakata, JM Pandolfi, GD Peterson, M Pratchett, T Ravasi, C Riginos, JL Rummer, B Schaffelke, T Wernberg, S Wilson. Regional Environmental Change. 2023, In press.
- Anemonefishes: A Model System for Evolutionary Genomics. M Herrera, T Ravasi, and V Laudet. F1000Research, 2023, 12:204, doi: 10.12688/f1000research.130752.1 (2023).
- The Clownfish Larvae Exhibit Faster Growth, Higher Metabolic Rates and Altered Gene Expression under future Ocean Warming. B Moore, J Jolly, M Izumiyama, E Kawai, T Ryu and T Ravasi. Science of the Total Environment 873, 2023, 162296, doi:10.1016/j.scitotenv.2023.162296, (2023).
- The chromosome-scale genome assembly of the yellowtail clownfish Amphiprion clarkii provides insights into melanic pigmentation of anemonefish. B Moore, M Herrera, E Gairin, C Li, S Miura, J Jolly, M Mercader, M Izumiyama, E Kawai, T Ravasi, V Laudet and T Ryu. G3 Genes|Genomes|Genetics, 2023, jkad002,doi:10.1093/g3journal/jkad002, (2023).
- Resilience and Adaptation to Local and Global Environmental Change. C Schunter, JM Donelson, PL Munday, T Ravasi. Evolution, Development and Ecology of Anemonefishes, 253-274, CRC Press, 2023.
- Transcriptome assemblies of two deep-sea octocorals Calyptrophora lyra and Chrysogorgia stellata from West Pacific seamount, Godin Guyot, T Ryu, S-J Hwang, S Woo, Marine Genomics 67, 2023.101006, doi: 10.1016/j.margen.2022.101006 (2023).
- Gobies inhabiting natural CO2 seeps reveal acclimation strategies to long-term acidification. S Suresh, A Mirasole, T Ravasi, S Vizzini, C Schunter. bioRxiv 2022.09.18.508416; doi: 10.1101/2022.09.18.508416 (2022).
- Monitoring threatened species with environmental DNA and open ecological data: Local distribution and habitat preferences of scalloped hammerhead sharks (Sphyrna lewini). AM Budd, T Schils, MK Cooper, MB Lyons, MS Mills, ME Deinhart, A Le Port, R Huerlimann, JM Strugnell, Biological Conservation 278,2023.109881, doi: 10.1016/j.biocon.2022.109881 (2022).
- The Interplay of Fungal and Bacterial Microbiomes on Rainforest Frogs Following a Disease Outbreak. DT McKnight, R Huerlimann, DS Bower, L Schwarzkopf, RA Alford, and KR Zenger, Ecosphere 13(7): e4037, doi: 10.1002/ecs2.4037 (2022)
- Anemonefish Genomics. N Salamin, C Schunter, A Monroe, T Ryu and T Ravasi. Evolution, Development and Ecology of Anemonefishes, 15-22, CRC Press, 2022.
- Neuro-molecular characterization of fish cleaning interactions. S Ramirez-Calero, JR Paula, E Otjacques, R Rosa, T Ravasi and C Schunter. Scientific Reports 12 (1), 8468, 2, (2022).
- Adaptation and Phenotypic Plasticity to Climate Change. T Ravasi, JM Donelson, JM Eirin-Lopez and LNS Shama. Front. Mar. Sci., Sec. Global Change and the Future Ocean, 9, doi: 10.3389/fmars.2022.893117 (2022).
- Parents exposed to warming produce offspring lower in weight and condition. RK Spinks, JM Donelson, LC Bonzi, T Ravasi and PL Munday. Ecol Evol. 17;12(7):e9044. doi: 10.1002/ece3.9044, (2022).
- Genetic architecture of behavioural resilience to ocean acidification. R Lehmann, C Schunter, MJ Welch, ST Arold, GE Nilsson, JN Tegner, PL Munday and T Ravasi. bioRxiv 2022.10.18.512656; doi: 10.1101/2022.10.18.512656, (2022).
- The alternative splicing landscape of a coral reef fish during a marine heatwave. SK Nok Chan, S Suresh, PL Munday, T Ravasi, MA Bernal, C Schunter. Ecology and Evolution.12(3) e8738, doi: 10.1002/ece3.8738, (2022).
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
1. Session Chair “Plasticity and adaptation of reef organisms to environmental change”. Asia-Pacific Coral Reef Symposium, Singapore, June 22nd, 2023.
2. Session Chair “What natural analogues teach us about the future of coral reefs”. Asia-Pacific Coral Reef Symposium, Singapore, June 19th, 2023.
3. “Adaptation and Acclimation of Coral Reef Fish as a Response to Climate Change”. New York University Center for Genomics and Systems Biology XI Symposium. Abu Dhabi, UAE, February 22nd, 2023.
4. “Adaptation and Acclimation of Coral Reef Fish as a Response to Climate Change”. Guest Lecture, University of Adelaide, Adelaide, Australia, February 8th, 2023
5. “Molecular basis and behavioural adjustments reveal potential local adaptation to acidifying oceans, a lesson from natural analogues.” The UNESCO Global Ocean Acidification Observing Network Seminar Series, Geneva, Switzerland, November 2nd, 2022.
6. “The Reefs of Okinawa”, IBM Tokyo Lab, Science of the Future. IBM Research, Tokyo, Japan, October 13, 2022.
7. “Genetic variation and phenotypic plasticity in the response of a coral reef fish to ocean acidification”. 5th International Symposium on the ocean in a high CO2 world, Lima, Peru, September 16th, 2022.
7. Session Chair “Can Global Change shape Adaptation and Acclimation of Coral Reef Species?”. 15th International Coral Reef Symposium (ICRS 2022), Bremen, Germany, July 5th, 2022.
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
-
[Seminar] Temperate carbonate reefs, 'blue carbon' and the 'plastisphere' by Prof. Jason Hall-Spencer, Universities of Plymouth (UK).
-
[Seminar] Enhancing detection of pest species using environmental DNA/RNA in biosecurity by: Dr Alejandro Trujillo-Gonzalez, Principal Scientist, National eDNA Reference Centre, University of Canberra, Canberra, ACT, Australia.
-
[Seminar] Comparative analysis of the cobia (Rachycentron canadum) genome identifies ephx1 as a novel putative master sex-determining gene in teleosts by: Dr Xueyan Shen, Senior Lecturer Aquaculture Institute, James Cook University Singapore.
-
[Seminar] The R&D pathway to growing aquaculture through innovative technologies by: Professor Dean R. Jerry, Tropical Futures Institute, James Cook University Singapore, Australian Research Council Hub for Supercharging Tropical Aquaculture through Genetic Solutions, James Cook University, Townsville, Australia.
-
[Seminar] When does taxonomy matter? by: Professor Andrew Baird, Chief Investigator in the ARC Centre of Excellence for Coral Reef Studies at James Cook University
-
[Seminar] The neurobiological effects of ocean acidification on a cephalopod by: Jodi Thomas, ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, QLD Australia.
7. Media outreach
-
- Fuji TV documentary-“Environmental Crisis: Islands of Grief and Collapsing Seas(環境クライシス嘆きの島々と崩壊する海). January 21, 2022. https://tver.jp/lp/episodes/epth4h0op7
-
This stunning Okinawan resort is working to save Japan's native Clownfish. Forbes, November 23, 2022. https://www.forbes.com/sites/jaredranahan/2022/11/23/this-stunning-okinawan-resort-is-working-to-save-japans-native-clownfish/?sh=3077580f3dc4
-
The clownfish could reveal new clues about climate adaptation. Earth.com, April 10, 2022 https://www.earth.com/news/the-clownfish-could-reveal-new-clues-about-climate-adaptation
7. Educational outreach
- Lecture by Erina Kawai, "Life of field work technician in Okinawa", at online event Lecture of vocation, OIST and Okinawa at Katsushika sogo high school.



