FY2013 Annual Report
Optical Neuroimaging Unit
Assistant Professor Bernd Kuhn


Abstract
In the FY 2013 the Optical Neuroimaging Unit continued several different in vivo imaging projects. Research projects focus on imaging neuronal activity in the mouse brain.
1. Staff
- Dr. Bernd Kuhn, Assistant Professor
- Dr. Sigita Augustinaite, Researcher
- Dr. Christopher J. Roome, Researcher
- Dr. Akihiro Funamizu, Researcher (shared with Doya Unit)
- Ryo Shiroishi, NAIST Master Student (shared with Doya Unit, until 8/2013)
- Dr. Kazuo Mori, Technical Assistant (from 3/2014)
- Dr. K. Kumkum Hossain, Technical Assistant (until 3/2014)
- Hiroko Chinone, Research Administrator
2. Collaborations
- Theme: Model-based decision making by two-photon microscopy
- Type of collaboration: Joint research
- Researchers:
- Professor Dr. Kenji Doya, Neural Computation Unit, OIST
- Dr. Akihiro Funamizu, Neural Computation Unit, OIST
- Ryo Shiroishi, Neural Computation Unit, OIST
- Theme: NMDA spikes in dendrites of thalamocortical neurons
- Type of collaboration: Joint research
- Researchers:
- Professor Dr. Paul Heggelund, University of Oslo, Norway
- Dr. P. Johannes Helm, University of Oslo, Norway
- Theme: Multi-photon excited luminescence of magnetic FePt core-shell nanoparticles
- Type of collaboration: Joint research
- Researcher:
- Dr. Klaus Seemann, Technical University Munich, Germany
- Theme: Two-photon imaging of ascidians
- Type of collaboration: Joint research
- Researcher:
- Professor Dr.Takeo Horie, Shimoda Marine Research Center, University of Tsukuba, Japan
- Theme: Dendritic diameters affect the spatial variability of intracellular calcium dynamics in computer models
- Type of collaboration: Joint research
- Researcher:
- Dr. Haroon Anwar, Computational Neuroscience Unit, OIST
- Hermina Nedelescu, Computational Neuroscience Unit, OIST
- Dr. Weiliang Chen, Computational Neuroscience Unit, OIST
- Prof. Dr. Erik De Schutter, Computational Neuroscience Unit, OIST
- Theme: Retrograde labeling of cortical neurons
- Type of collaboration: Joint research
- Researcher:
- Professor Dr.Kazuto Kobayashi, Fukushima Medical University, Japan
- Professor Dr. Shigeki Kato, Fukushima Medical University, Japan
3. Activities and Findings
3.1 In vivo imaging in barrel cortex of mouse
In the FY2013 we continued optimized surgical procedures and in vivo imaging techniques. One of the optimizations resulted in a patent. We focus on barrel cortex and visual cortex.
3.2 Investigation of action-dependent state prediction by two-photon microscopy
In uncertain and changing environments, we sometimes cannot get enough sensory inputs to know the current context, and therefore must infer the context with limited information to make a decision. We call such internal simulation of context a mental simulation. One illustrative example of the mental simulation happens in a game called watermelon cracking, in which a person tries to hit a watermelon far away with his eyes closed with a stick: the person needs to estimate his position based on his actions and sensory inputs, e.g., words from others. To investigate the neural substrate of mental simulation, we use two-photon fluorescence imaging in awake behaving mice which enables us to simultaneously image multiple identified neurons and their activities while the mouse conducts a task.
A mouse was head restrained and maneuvered a spherical treadmill. 12 speakers around the treadmill provided a virtual sound environment. The direction and the amplitude of sound pulses emulated the location of sound source, which was moved according to the mouse’s locomotion on the treadmill. When the mouse reached the sound source and licked a spout, it got a water reward. In some trials, the sound was intermittently omitted: the mouse asked to utilize a mental simulation to reach the sound source.
Calcium is an important second messenger in neurons and an increased calcium concentration is correlated with neuronal activity. For this reason we use a genetically encoded calcium indicator, called G-CaMP, in posterior parietal cortex (PPC) of mice to detect neuronal activities. We could record the activities (i) of more than 500 neurons simultaneously and (ii) of cortical layer 1 to 5 for one hour continuously (Figure 1, 2). From the population activities in layer 2, we decoded the distance to sound source by the least absolute shrinkage and selection operator (LASSO). LASSO extracted the relevant neurons for distance coding; the neurons were homogeneously distributed in the PPC. Also, the decoder could successfully decode the sound-source distance during no-sound periods. These results suggest that PPC neurons represent and update the distance of sound source not only from present auditory inputs but also by dynamic update of the estimate using an action-dependent state transition model.
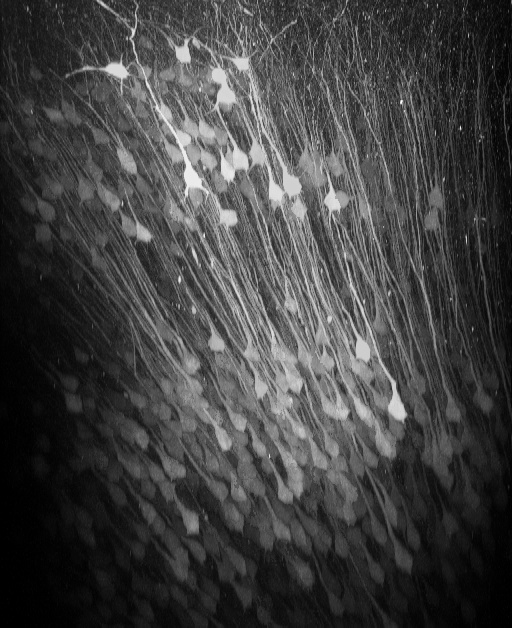
Figure 1: In vivo two-photon reconstruction of neurons infected by AAV2/1-hSYN-GCaMP6f in posterior parietal cortex. Layer 2/3 and layer 5 neurons express the genetically encoded calcium indicator GCaMP6f.
Figure 2: (A) In-vivo imaging of neurons with two-photon microscopy. The image shows the xz plane reconstruction of calcium-sensor-expressing cortical neurons in posterior parietal cortex . The dotted line indicates the imaging plane in (B). Neurons coding sound-source distance were extracted by LASSO. 282 neurons colored red or blue were found among 540 neurons (B). The color depth shows the weights in LASSO. (C) Distance estimation extracted from neuronal activity with LASSO. Horizontal and vertical axes show the actual and estimated distance to sound source, respectively. The estimations were successful even during the no-sound periods in the intermittent condition.
3.3 NMDA spike/plateau potentials in dendrites of thalamocortical neurons
We were studying the visual pathway – the pathway which sends signals from the eye to the cerebral cortex, where the visual information is processed. Between the ganglion cells of the eye and the cortex there is one more station which the information has to pass: The visual thalamus. The thalamus is thought of being a relay which can gate the visual information or block it. For example, when we are awake we see our environment but if we are sleeping this visual input to our brain is turned off. We try to understand how this switching could occur.
The relay neurons in the thalamus are called thalamocortical (TC) neurons because they send the signal which they get from the retina from thalamus to cortex. Interestingly, the dendrites of TC neurons get a second input coming from the cortical layer 6.
To understand what this input from cortex is good for, we did experiments with brain slices and emulated the cortical input to the TC neurons by local glutamate application (Figure 3). We found that the glutamate application causes a dendritic activation, so called NMDA spike/plateau potentials (Figure 3, 4). Interestingly, this activation in the dendrite switches the mode of the neuron and now allows the information to pass (Figure 5,6). Without the dendritic activation the pathway is blocked. We were also able to find out how this “change of mode” works: T-type calcium channels cannot recover from inactivation when dendrites are activated. Without T-type calcium channels the neurons will not burst but reliably gate signals from retina.
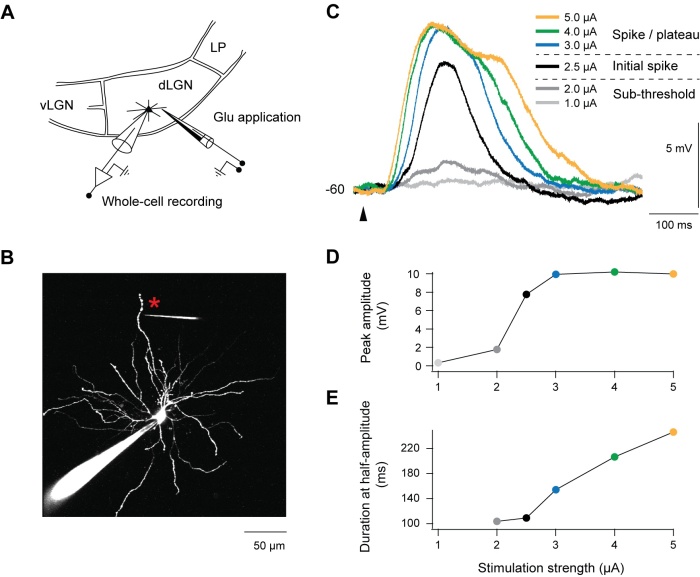
Figure 3: Dendrites of TC neurons in visual thalamus (dLGN) are stimulated by glutamate application (A,B). With increasing glutamate application the electrical recordings from the soma of TC neurons show a step like increase in amplitude (C,D) and a plateau potential with increasing duration (C,E).
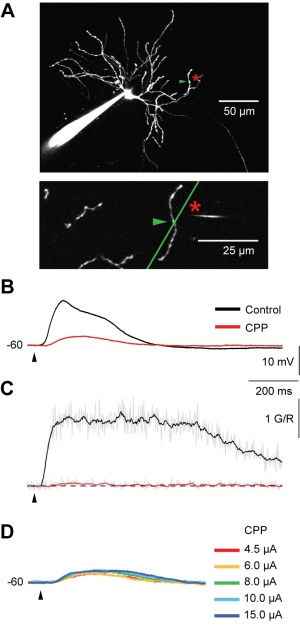
Figure 4: The spike/plateau potentials are mediated by NMDA receptors as can be shown by pharmacology (CPP application) and two-photon imaging. A TC neuron (A) is stimulated by glutamate application to the dendrite (location indicated by asterix). Simultaneous electrical (B) and optical recording (two-photon calcium recordings, C) show dendritic spike/plateau potentials which are almost completely blocked by CPP, a NMDA receptor antagonist. Also strong glutamate application is not able to recover the potential (D).
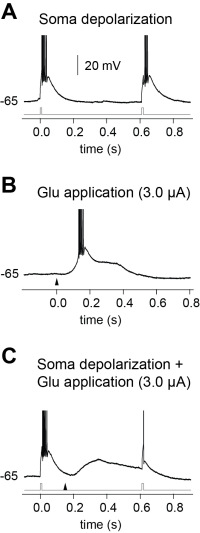
Figure 5: (A) Electrical stimulation of a TC neuron at the soma without NMDA receptor activation results in bursting. (B) Glutamate stimulation results in a NMDA-mediated plateau. (C) Combined electrical (gray trace ) and glutamate (triangle) stimulation results in a single action potential. This shows that NMDA spike/plateau potentials switch the mode of TC neurons from bursting to regular spiking.
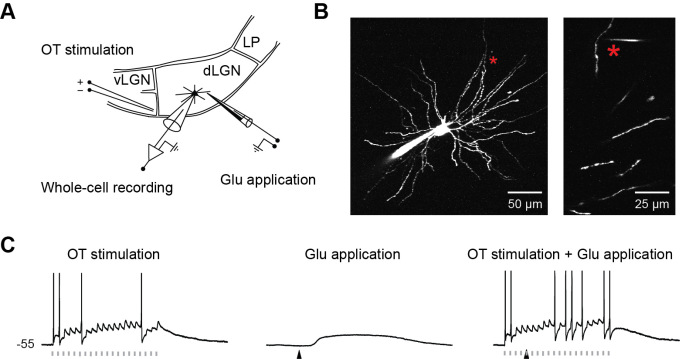
Figure 6: (A, B) TC neurons are stimulated by glutamate application to the distal dendrites and by activation of axons coming from retina, called optical tract. Optical tract stimulation of a slightly depolarized neuron results in unreliable action potential generation (C, left). Glutamate stimulation results in a plateau potential in the dendrite (not shown) and soma (C, middle). Stimulation of the optical tract during a plateau potential strongly increases the reliability of action potential transmission (C, right). This shows that NMDA spike/plateau potentials are needed for reliable gating of information coming from ganglion cells of the retina.
3.4 Multi-photon excited luminescence of magnetic FePt core-shell nanoparticles
Magnetic nanoparticles are promising tools for fighting cancer through hypothermia treatment.
We study magnetic FePt nanoparticles (Figure 7) with a hydrophilic, inert, and biocompatible silico-tungsten oxide shell. The particles can be functionalized, optically detected, and optically manipulated. To show the functionalization the fluorescent dye NOPS was bound to the FePt core-shell nanoparticles with propyl-triethoxy-silane linkers and the labeled particles were observed in ethanol (EtOH). In aqueous dispersion the NOPS fluorescence is quenched making them invisible using 1-photon excitation. However, we observe bright luminescence of labeled and even unlabeled magnetic core-shell nanoparticles with multi-photon excitation. Luminescence can be detected in the near ultraviolet and the full visible spectral range by near infrared multi-photon excitation (Figure 8). For optical manipulation, we were able to drag clusters of particles, and maybe also single particles, by a focused laser beam that acts as optical tweezers by inducing an electric dipole in the insulated metal nanoparticles. In a first application, we show that the luminescence of the core-shell nanoparticles is bright enough for in vivo multi-photon imaging in the mouse neocortex down to cortical layer 5 (Figure 9, 10).
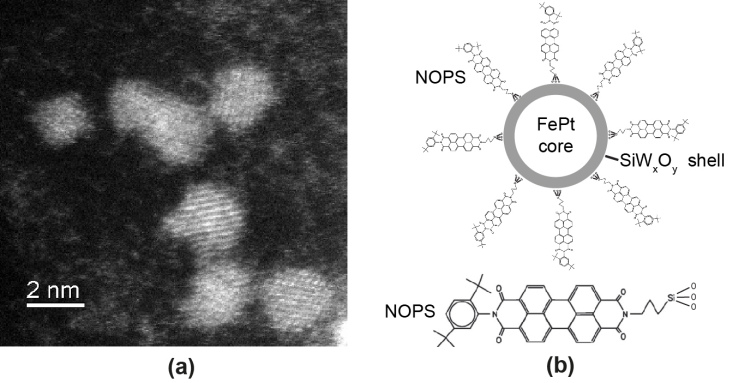
Figure 7: HR-TEM image of magnetic FePt core-shell nanoparticles, however, the shell is not visible without averaging (a). Tungsten oxide covering the surface of the crystalline nanoparticles causes dispersibility in polar solvents like EtOH and water but also allows functionalization as, for example, labeling with the fluorescence dye NOPS as illustrated schematically in (b).
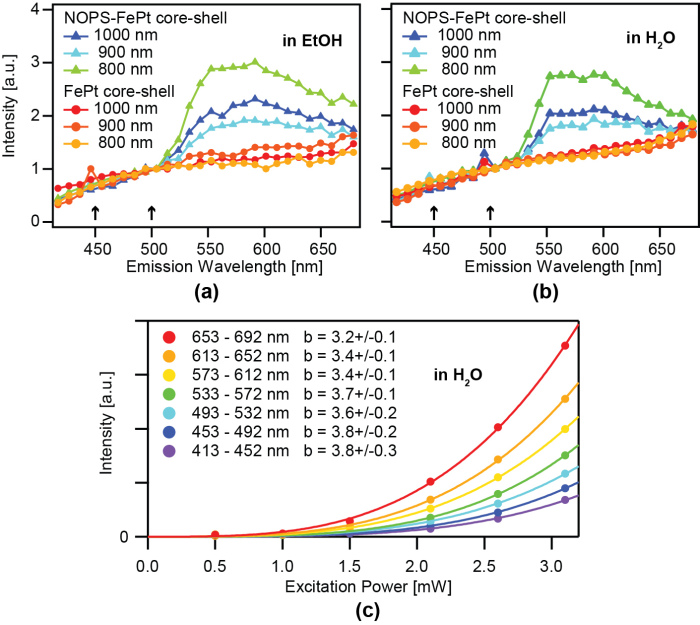
Figure 8: Average multi-photon-excited fluorescence/luminescence spectra of NOPS-labeled and unlabeled magnetic FePt core-shell particles immobilized on glass slides in EtOH (a) and H2O (b) excited with 1.5 mW at 800 nm, 900 nm, 1000 nm. Spectra were normalized to the intensity at 507 nm. The difference of the two spectra resembles the emission spectrum of NOPS but shows significant differences compared to the 1-photon emission spectrum in EtOH (Fig. 2). In some spectra the backscattered or reflected second harmonic signal is visible (arrows). The intensity of unlabeled magnetic FePt core-shell nanoparticles, immobilized on a glass slide with water added and excited at 1000 nm, increases wavelength-dependent by the power of 3.8 to 3.2 up to an excitation power of 3.2 mW (c). Background-corrected data was fitted by y(x) = axb. Values of b are listed ±standard deviation.
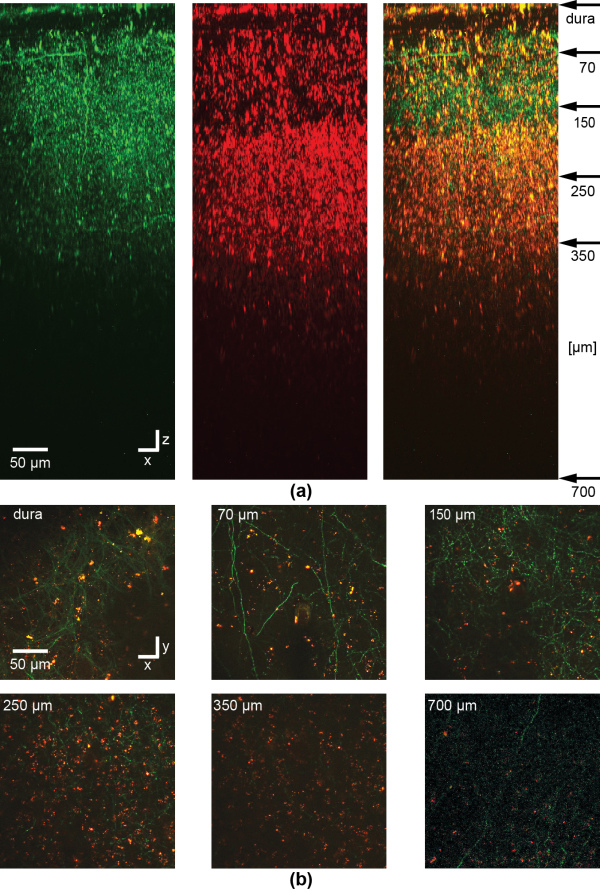
Figure 9: In vivo imaging of NOPS-labeled magnetic nanoparticles or clusters of particles in the barrel cortex of an anesthetized mouse. To label a subset of neurons in barrel cortex, at first, a viral vector is injected delivering the DNA of the fluorescent protein eGFP. 4 days later, magnetic FePt nanoparticles were injected and imaged through a chronic cranial window with multi-photon microscopy in the mouse barrel cortex. A stack of images was recorded with two channels starting from the dura and then reconstructed to show the side view (a). Clusters of NOPS-labeled magnetic FePt core-shell nanoparticles and maybe also single particles are visible in the green (left) and red (middle) channel while the axons and dendrites of eGFP expressing neurons are only visible in the green, as can be seen in the overlay (right). Particles or clusters of particles can be detected in xy images between axons (majority of processes) and dendrites down to a depth of 700 µm (b). Depth of imaging is indicated in the upper left corner corresponding to the arrows in (a). For the image at 700 µm in (b) the contrast and averaging was increased in comparison to the images at lower depth. The dura is visible due to backscattering of the second harmonic signal of collagen. The excitation wavelength was 1000 nm.
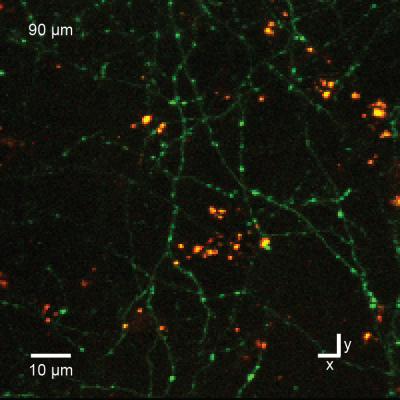
Figure 10: In vivo imaging as in Fig.7 but at higher magnification shows nanoparticles (yellow) and axons of cortical pyramidal neurons (green) in cortical layer 1, 90 µm below the brain surface. The bright green spots along the axons are synaptic boutons.
3.5 Two-photon imaging of ascidians
We image the larvae of transgenic ascidians with two-photon microscopy. This allows us to get higher resolution than with confocal microscopy due to lower scattering artefacts. We studied morphology in fixed larvae (Figure 11) and functional signals in vivo.
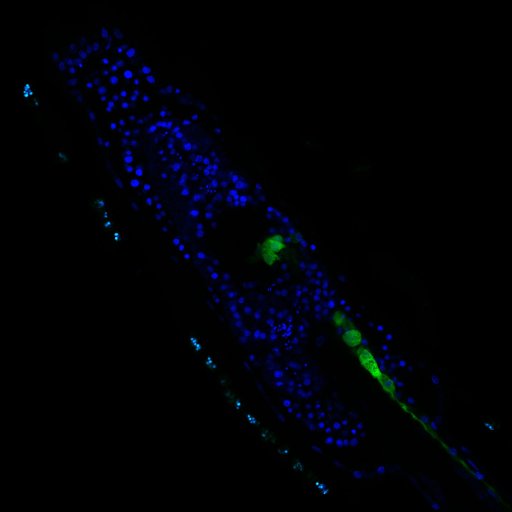
Figure 11: Transgenic ascidian larvae with DAPI labeling of cell nuclei (blue) and cholinergic neurons (green) imaged with two-photon microscopy.
3.6 Retrograde labeling of cortical neurons
We are exploring the possibilities of retrograde labeling of neurons for functional imaging. We are testing different designs of viral vector systems.
3.7 Dendritic diameters affect the spatial variability of intracellular calcium dynamics in computer models
The Optical Neuroimaging Unit supported this project of the Computational Neuroscience Unit by experimental data.
There is growing interest in understanding calcium dynamics in dendrites, both experimentally and computationally. Many processes influence these dynamics, but in dendrites there is a strong contribution of morphology because the peak calcium levels are strongly determined by the surface to volume ratio of each branch, which is inversely related to branch diameter. In this project we explore the predicted variance of dendritic calcium concentrations due to local changes in dendrite diameter and how this is affected by the modeling approach used. We investigate this in a model of dendritic calcium spiking in different reconstructions of cerebellar Purkinje cells (Figure 12) and in morphological analysis of neocortical and hippocampal pyramidal neurons.
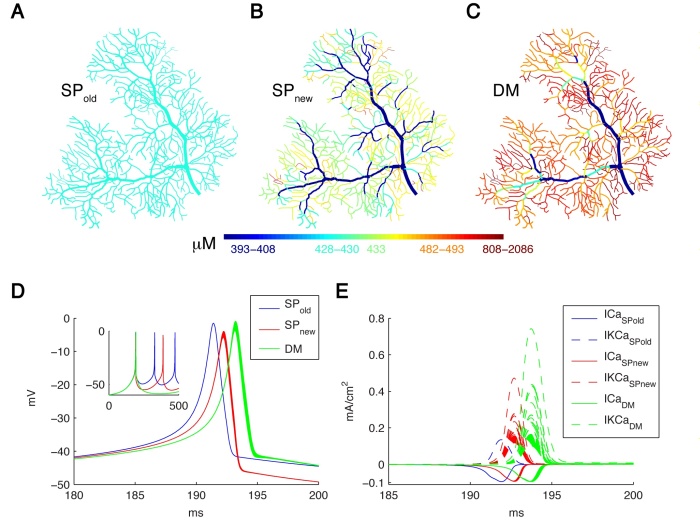
Figure 12: Spatial Ca2+ gradients strongly depend on the type of model implementation. Panels A-C show maps of the integrated calcium levels in the dendrite during a spontaneous burst of Ca2+ spikes (panel D). The dendritic branches are color coded to show the integrated calcium levels using a 20 ms window around the peak Ca2+ concentration of the first dendritic Ca2+ spike. The color scales used in these maps are nonlinear (using histogram equalization) to enhance the contrast. (A) Single Ca2+ pool model using SPold mechanism results in homogenous Ca2+ levels. (B) Single Ca2+ pool model using SPnew mechanism results in variable Ca2+ levels. (C) Detailed Ca2+ dynamics model with buffering and 1D diffusion results in variable Ca2+ levels with larger Ca2+ gradients. (D) Voltage traces show the first spike of the Ca2+ burst for each model in all dendritic compartments for the 3 different models (see color code in Figure). The inset shows complete traces. (E) The underlying Ca2+ and KCa currents (recorded from all dendritic compartments) for the Ca2+ spike of the 3 different models (see color code in Figure).
3.8 Physiological temperature during brain slicing enhances the quality of acute slice preparations
In an independent project Shiwei Huang (Computational Neuroscience Unit) and Marylka Y. Uusisaari work on brain dissection and slicing using solutions warmed to near-physiological temperature (~ +34°C). This greatly enhances slice quality without affecting intrinsic electrophysiological properties of the neurons. Improved slice quality is seen not only when using young (<1 month), but also mature (>2.5 month) mice. This allows easy in vitro patch-clamp experimentation using adult deep cerebellar nuclear slices, which until now have been considered very difficult. As proof of the concept, Shiwei and Marylka compare intrinsic properties of cerebellar nuclear neurons in juvenile (<1 month) and adult (up to 7 months) mice, and confirm that no significant developmental changes occur after the fourth postnatal week. The enhanced quality of brain slices from old animals facilitates experimentation on age-related disorders as well as optogenetic studies requiring long transfection periods (Figure 13).
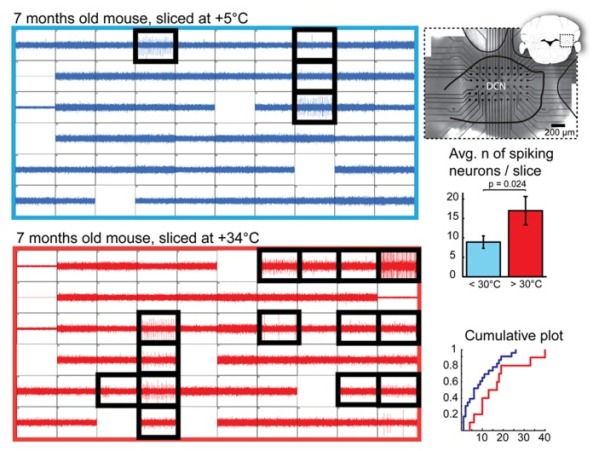
Figure 13: Quantification of Deep Cerebellar Nuclei (DCN) slice healthiness with multi-electrode array recordings. Left: representative recordings with 60 planar extracellular electrodes from 7-month old mice from DCN slices prepared at cold (top, blue) and warm (bottom, red) temperatures. Each grid square represents 1 second of continuous recording (verticalaxis: 100pA); blank squares are electrodes with degraded recording quality. Electrodes in which spiking cells were detected are indicated with black, thick squares. A micro graph combined with a schematic drawing of a coronal cerebellar slice (right top panel) shows positioning of the multi-electrode array within the DCN. Right, middle and bottom panels: Comparisons of average and cumulative sums of the numbers of spiking cells detected per slice in < +30◦C (+5 to 28◦C, blue) and +30 to +34◦C (red) conditions shows that when slices are prepared at warm temperatures, the probability of finding patchable neurons is increased. N = 23 (seven animals) slices cut in cold conditions, 10 (four animals) in warm.
4. Publications
4.1 Journals
- Huang, S. & Uusisaari, M. Y. Physiological temperature during brain slicing enhances the quality of acute slice preparations. Frontiers in Cellular Neuroscience 7, 1-8, doi:doi: 10.3389/fncel.2013.00048 (2013).
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Augustinaite, S., Kuhn, B., Helm, P. J. & Heggelund, P. NMDA spikes in dendrites of thalamocortical neurons, in Society for Neuroscience 2013, San Diego, CA, U.S.A. (2013).
- Augustinaite, S., Kuhn, B., Helm, P. J. & Heggelund, P. Presentation Signal transduction and modulation, in Neuro2013, Kyoto, Japan (2013).
- Funamizu, A., Kuhn, B. & Doya, K. Investigation of neuronal activity in the posterior parietal cortex by two-photon microscopy, in International symposium on Prediction and Decision Making, Kyoto, Japan (2013).
- Hamada, H., Kuhn, B. & Arbuthnott, G. Finding active projections in a terminal system, in Imaging the brain at different scales: How to integrate multi-scale structural information? Antwerp, Belgium (2013).
- Nedelescu, H., Arbuthnott, G., Kuhn, B., De Schutter, E., Yarom, Y. & Uusisaari, M. Y. Visualization of the Purkinje neuron to cerebellar nuclei anatomical connectivity using a viral approach, in Society for Neuroscience 2013, San Diego, CA, U.S.A. (2013).
5. Intellectual Property Rights and Other Specific Achievements
- Kuhn, B. & Roome, C. J. Chronic Cranial Window Allowing Drug Application and Electrophysiology. (2014).
6. Meetings and Events
6.1 Workshop: Okinawa Computational Neuroscience Course 2013
- Date: June 17- July 4, 2013
- Venue: OIST Seaside House
- Organizers: Drs. E. De Schutter, K. Doya, J. Wickens
- Speaker (among others): Bernd Kuhn
- Voltage-gated channels and the Hodgkin-Huxley model of neuronal activity
- Optical Imaging Methods
6.2 Onna-son/OIST Children’s School of Science 2013
- Date: June 17- July 4, 2013
- Venue: Fureai Taiken Center
- Organizer: Prof. M.A. Price
- Speaker (among others): Bernd Kuhn
- Palaeontology of Okinawa, Grade 1-3 (Figure 14)

Figure 14: Fossil Maple Leaf Triton (Biplex sp.) of Okinawa. The shell was found in late Tertiary mudstone (about 2 M years old).



