FY2012 Annual Report
Optical Neuroimaging Unit
Assistant Professor Bernd Kuhn


Abstract
In the FY 2012 the Optical Neuroimaging Unit completed the lab setup with two two-photon setups for in vivo imaging. Research projects started focusing on imaging neuronal activity in vivo.
1. Staff
- Dr. Bernd Kuhn, Assistant Professor
- Dr. Sigita Augustinaite, Researcher
- Dr. Christopher J. Roome, Researcher
- Dr. Marylka Y. Uusisaari, Researcher
- Dr. Akihiro Funamizu, Researcher (shared with Doya Unit)
- Ryo Shiroishi, Graduate student (shared with Doya Unit)
- Dr. K. Kumkum Hossain, Research Assistant
- Hiroko Chinone, Research Administrator
2. Collaborations
- Theme: Investigation of model-based decision making by two-photon microscopy
- Type of collaboration: Joint research
- Researchers:
- Professor Dr. Kenji Doya, Neural Computation Unit, OIST
- Dr. Akihiro Funamizu, Neural Computation Unit, OIST
- Ryo Shiroishi, Neuroal Computation Unit, OIS
- Theme: NMDA spikes in dendrites of thalamocortical neurons
- Type of collaboration: Joint research
- Researchers:
- Dr. Sigita Augustinaite, University of Oslo, Norway; since Oct. 1, 2012, OIST
- Professor Dr. Paul Heggelund, University of Oslo, Norway
- Dr. P. Johannes Helm, University of Oslo, Norway
- Theme: Combinatorial genetic approach for cell type specific expression of fluorescent calcium indicator proteins
- Type of collaboration: Joint research
- Researchers:
- Professor Dr. Ilker Ozden, Brown University, U.S.A.
- Yulia Lampi, Princeton University, U.S.A.
- Dr. Mazahir T. Hasan, Max-Planck-Institute for Medical Research, Germany
- Professor Dr. Samuel S.-H. Wang, Princeton University, U.S.A.
- Theme: pH measurement within protein crystals
- Type of collaboration: Joint research
- Researchers:
- Dr. Klaus M. Seemann, Peter Grunberg Institute, Research Center Julich, Germany
- Dr. Reiner Kiefersauer, Proteros biostructures GmbH, Martinsried, Germany
- Prof. Dr. Uwe Jacob, Westend-Innovation GmbH, Munchen, Germany
- Theme: 2-photon imaging of ascidians
- Type of collaboration: Joint research
- Researcher:
- Dr.Takeo Horie, Shimoda Marine Research Center, University of Tsukuba, Japan
3. Activities and Findings
3.1 In vivo and in vitro imaging in barrel cortex of mouse
In the FY2012 we finalized the setup process for in vitro and in vivo brain imaging. We optimized surgical procedures and imaging techniques. We focus on barrel cortex.
3.2 An amplified promoter system for targeted expression of calcium indicator proteins
Recording of identified neuronal network activity using genetically encoded calcium indicators (GECIs) requires labeling that is cell type-specific and bright enough for the detection of functional signals. However, specificity and strong expression are often not easily achieved using the same promoter. Here we present an inducible combinatorial approach for targeted expression and single-cell-level quantification in which a specific weak promoter is used to drive trans-amplification under a strong general promoter. We demonstrated this approach using recombinant adeno-associated viruses (rAAVs) to deliver the sequence of the GECI D3cpv in the mouse cerebellar cortex. Direct expression under the human synapsin promoter (hSYN) led to high levels of expression (50-100 µM) in all five interneuron types of the cerebellar cortex but not in Purkinje cells (PCs) (~10 μM), yielding sufficient contrast to allow functional signals to be recorded from somata and processes in awake animals using two-photon microscopy. When the hSYN promoter was used to drive expression of the tetracycline transactivator (tTA), a second rAAV containing the bidirectional TET promoter (Ptetbi) could drive strong D3cpv expression in PCs (~100 µM), enough to allow reliable complex spike detection in the dendritic arbor. An amplified approach should be of use in monitoring neural processing in selected cell types and to boost expression of optogenetic probes. Additionally, we overcome cell toxicity associated with local GECI overexpression by combining the virus injection with systemic pre-injection of hyperosmotic D-mannitol, and by this double the time window for functional imaging.
http://www.frontiersin.org/neural_circuits/10.3389/fncir.2012.00049/abstract
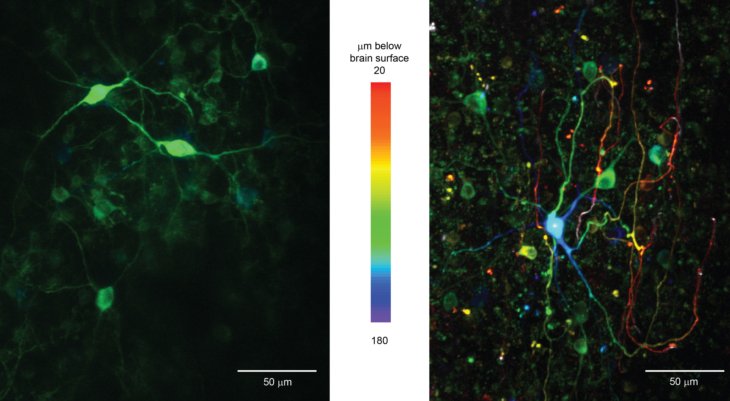
Figure: Cerebellar interneurons like Lugaro (left) and Golgi (right) cells can be specifically labeled and used for functional imaging in vivo with genetically encoded calcium indicators by using the human synapsin promoter.
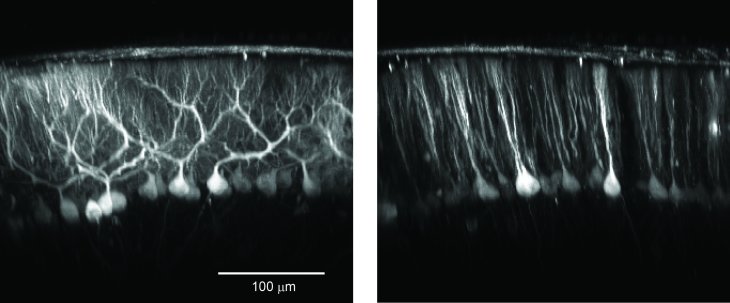
Figure: Cerebellar Purkinje cells can be specifically labeled and used for functional imaging in vivo with genetically encoded calcium indicators by using thethe TET promoter system.
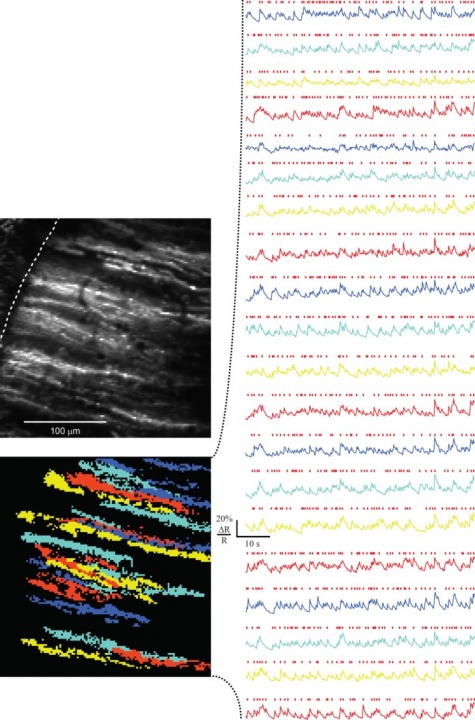
Figure: Functional imaging of dendrites of cerebellar Purkinje cell in awake mice.
3.3 Optical pH detection within a protein crystal
The pH is one of the key parameters governing protein conformation and activity. In protein crystals, however, the pH is so far not accessible by experiment. Here, we report on the optical detection of the pH in a lysozyme crystal employing the pH-sensitive fluorescent dyes SNARF-1 and SNARF-4F. The molecular probes were loaded into the crystal by diffusion. Two-dimensional fluorescence spectra of the labeled protein crystal were recorded and the average pH of the crystal at different bath pH was determined by calibrating fluorescence peak ratios. In addition, we used two-photon microscopy to spatially resolve the pH inside a lysozyme crystal three-dimensionally and to follow pH changes in response to a pH change of the bath over time. At equilibrium at bath pH between 5.5 and 8.0 we found a pH in the water-filled crystal channels that was DpH = -0.3 to -1.0 lower than that of the bath. This corresponds to a 2 to 10-fold higher proton concentration in the crystal channels than in the bath. The lower pH at equilibrium in the crystal channels can be explained by slower proton diffusion in the channels than in the bath and a resulting proton accumulation in the crystal for conservation of mass and so an equilibrium of flux.
http://pubs.acs.org/doi/abs/10.1021/jp2103512
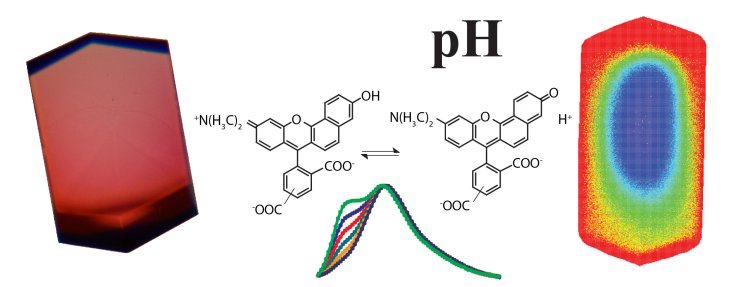
Figure: The pH sensitive fluorescent dye NARF-1 (center) brightly labels lysozym crystals (left) and allowas us to measure pH and pH changes in the crystal (right).
3.4 Investigation of model-based decision making by two-photon microscopy
In uncertain and changing environments, we sometimes cannot get enough sensory inputs to know the current context, and therefore must infer the context with limited information to make a decision. We call such internal simulation of context a mental simulation. One illustrative example of the mental simulation happens in a game called watermelon cracking, in which a person tries to hit a watermelon far away with his eyes closed with a stick: the person needs to estimate his position based on his actions and sensory inputs, e.g., words from others. To investigate the neural substrate of mental simulation, we use two-photon fluorescence imaging in awake behaving mice which enables us to simultaneously image multiple identified neurons and their activities while the mouse conducts a task.
Calcium is an important second messenger in neurons and an increased calcium concentration is correlated with neuronal activity. For this reason we use a genetically encoded calcium indicator, called G-CaMP, in secondary motor cortex (M2) of mice to detect neuronal activity. When a neuron becomes active, G-CaMP will become brighter. Figures show the two-photon image of neurons labeled with G-CaMP in an anesthetized mouse, indicating that neurons in layer 2/3 were successfully labeled. In preliminary experiments we conducted a simple Pavlovian conditioning task and functionally imaged neuronal activity in awake behaving mice. In this task, mice get a water reward after a tone presentation. We found that layer 2/3 neurons in M2 showed correlated activities with tones, licking, and locomotion, and that such characterized neurons were homogeneously distributed in space.
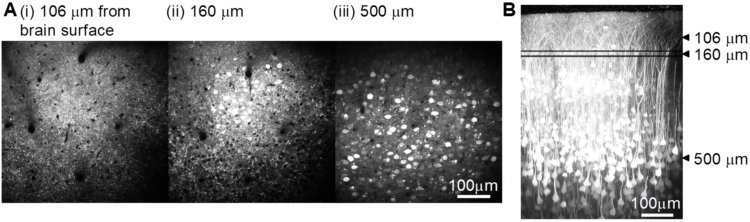
Figure: In vivo imaging of neurons with two-photon microscopy. Images show layer 2/3 neurons through a chronic cranial window in the xy plane (A, i - iii) at different depth below the brain surface and a reconstructed view of the xz plane (B).
3.5 2-photon imaging of ascidians
We image the lavae of ascidians with two-photon microscopy. This allows us to get higher resolution than with confocal microscopy due to lower scattering artefacts.
3.6 NMDA spikes in dendrites of thalamocortical neurons
We studied dendritic signal processing in TC neurons with combined two-photon calcium imaging and somatic whole-cell recording of responses to local dendritic glutamate stimulation.
3.7 Physiological temperature during brain slicing enhances the quality of acute slice preparations
In an independent project Shiwei Huang (Computational Neuroscience Unit) and Marylka Y. Uusisaari work on brain dissection and slicing using solutions warmed to near-physiological temperature (~ +34°C). This greatly enhances slice quality without affecting intrinsic electrophysiological properties of the neurons. Improved slice quality is seen not only when using young (<1 month), but also mature (>2.5 month) mice. This allows easy in vitro patch-clamp experimentation using adult deep cerebellar nuclear slices, which until now have been considered very difficult. As proof of the concept, they compare intrinsic properties of cerebellar nuclear neurons in juvenile (<1 month) and adult (up to 7 months) mice, and confirm that no significant developmental changes occur after the fourth postnatal week. The enhanced quality of brain slices from old animals facilitates experimentation on age-related disorders as well as optogenetic studies requiring long transfection periods.
4. Publications
4.1 Journals
- Kuhn B., Ozden I., Lampi Y., Hasan M.T., Wang S.S.-H. An amplified promoter system for targeted expression of calcium indicator proteins in the cerebellar cortex. Frontiers in Neuronal Circuits 2012(6):49 (2012)
-
Seemann K.M., Kiefersauer R., Jacob U., Kuhn B. Optical pH Detection within a Protein Crystal. J. Phys. Chem. B 116(33): 9873-9881 (2012)
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Funamizu A., Kuhn B., Doya K. Investigation of neuronal activities in secondary motor cortex by two-photon microscopy Winter Workshop „Mechanisms of Brain and Mind – Pleasure and Pain“, Hokkaido, Japan, January 9-11, 2013
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 Workshop: Okinawa Computational Neuroscience Course 2012
- Date: June 10-29, 2012
- Venue: OIST Seaside House
- Organizers: Drs. E. De Schutter, K. Doya, J. Wickens
- Speaker (among others): Bernd Kuhn
6.2 Workshop: Laser Bio Medical Technical Meeting
- Date: December 7, 2012
- Venue: Ryukyu University
- Organizer: Prof. Y. Oki
- Speaker (among others): Bernd Kuhn
6.3 JST/CREST Meeting
- Date: December 3, 2012
- Venue: OIST
- Organizer: JST/CREST
- Speaker (among others): Bernd Kuhn
6.4 Partial Solar Eclipse
- Date: May 21, 2012
- Venue: OIST

6.5 Transit of Venus in front of the sun
- Date: June 7, 2012
- Venue: OIST




