FY2015 Annual Report
Computational Neuroscience Unit
Professor Erik De Schutter 
Abstract
We use computational, data-driven methods to study how neurons and microcircuits in the brain operate. We are interested in how fundamental properties, such as a neuron’s morphology and its excitability, interact with one another during common neural functions like information processing or learning. Most of our models concern the cerebellum as this brain structure has a relatively simple anatomy and the physiology of its main neurons has been studied extensively, allowing for detailed modeling at many different levels of complexity.
1. Staff
Molecular modeling
- Iain Hepburn, Technical Staff
- Andrew Gallimore, Researcher (from May 2015)
- Himanshu Gangal, Technical Staff (till June 2015)
- Nikon Rasumov, Researcher (till July 2015)
- Criseida Zamora, Researcher (from July 2016)
Cellular modeling
- Sungho Hong, Group Leader
- Akira Takashima, Researcher (till February 2016)
- Yunliang Zang, Researcher
Network modeling
- Benjamin Torben-Nielsen, Group Leader (till October 2015)
- Tom Close, Researcher (till June 2015)
- Sergio Verduzco, Researcher (from November 2015)
- Shyam Kumar Sudhakar, Special Research Student (till December 2015)
Software development
- Weiliang Chen, Researcher
- Guido Klingbeil, Researcher (from July 2015)
Visiting Researcher
- Marylka Yoe Uusisaari (from January 2016)
Research Interns
- Russell Jarvis, Research Intern (till June 2015)
- Chihiro Kurosawa, Research Intern (till April 2015)
- Aleix Boquet Pujadas, Research Intern (from September till November 2015)
Rotation Students
- Sergey Zobnin, Student (Term1)
- Ratnesh Kumar Gupta. Student (Term2)
Research Unit Administrator
- Sachie Matsuoka
2. Collaborations
- Theme: Cerebellar physiology, multiple themes
- Type of collaboration: Scientific collaboration and graduate program
- Researchers:
- Professor M. Giugliano, University of Antwerp, Belgium
- Professor D. Snyders, University of Antwerp, Belgium
- Joao Couto, University of Antwerp, Belgium
- Q. Robberecht, University of Antwerp, Belgium
- Theme: Spiking activity of monkey cerebellar neurons
- Type of collaboration: Scientific collaboration
- Researchers:
- Professor H.P. Thier, University of Tübingen, Germany
- A. Ignashchenkova, University of Tübingen, Germany
- Dr. M. Junker, University of Tübingen, Germany
- A. Schmigdlin, University of Tübingen, Germany
- Theme: Human Brain Project: simulator development
- Type of collaboration: Scientific collaboration
- Researchers:
- Prof. F. Schürmann, École Polytechnique Fédérale de Lausanne, Switzerland
- F. Delalondre, École Polytechnique Fédérale de Lausanne, Switzerland
- Theme: Molecular identification of cerebellar signaling pathways and cerebellar optogenetics
- Type of collaboration: Scientific collaboration
- Researchers:
- Professor K. Tanaka, Korea Institute for Science and Technology (KIST), Korea
- Professor K. Tanaka, Korea Institute for Science and Technology (KIST), Korea
- Theme: Modeling of effects of ethanol on the cerebellum
- Type of collaboration: Joint research
- Researchers:
- Professor C.F. Valenzuela, University of New Mexico, United States of America
- Professor C.F. Valenzuela, University of New Mexico, United States of America
- Theme: In vivo cerebellar activity
- Type of collaboration: Scientific collaboration
- Researchers:
- Professor C. De Zeeuw, Erasmus Medical Center, Rotterdam, The Netherlands
- Professor L.W.J. Bosman, Erasmus Medical Center, Rotterdam, The Netherlands
- Dr. M. Negrello, Erasmus Medical Center, Rotterdam, The Netherlands
- Theme: Purkinje cell morphology and physiology, modeling
- Type of collaboration: Scientific collaboration
- Researchers:
- Professor M. Häusser, University College London, United Kingdom
- Professor A. Roth, University College London, United Kingdom
- Dr. S. Dieudonné, Ecole Normale Supérieure, Paris, France
3. Activities and Findings
Cellular mechanisms regulating firing and synaptic properties of neurons
Leak currents in Purkinje cells are non-linear
In their seminal works on squid giant axons, Hodgkin, and Huxley approximated the membrane leak current as Ohmic, i.e., linear, since in their preparation, sub-threshold current rectification due to the influence of ionic concentration is negligible. Most studies on mammalian neurons have made the same, largely untested, assumption. Using simulation methods we have shown that the membrane time constant and input resistance of mammalian neurons (when other major voltage-sensitive and ligand-gated ionic currents are discounted) varies non-linearly with membrane voltage, following the prediction of a Goldman-Hodgkin-Katz (GHK) based passive membrane model (Huang et al., 2015). The model predicts that under such conditions, the time constant/input resistance-voltage relationship will linearize if the concentration differences across the cell membrane are reduced. These predictions were confirmed in patch-clamp recordings of cerebellar Purkinje neurons (in the presence of pharmacological blockers of other background ionic currents) and were more prominent in the sub-threshold region of the membrane potential.
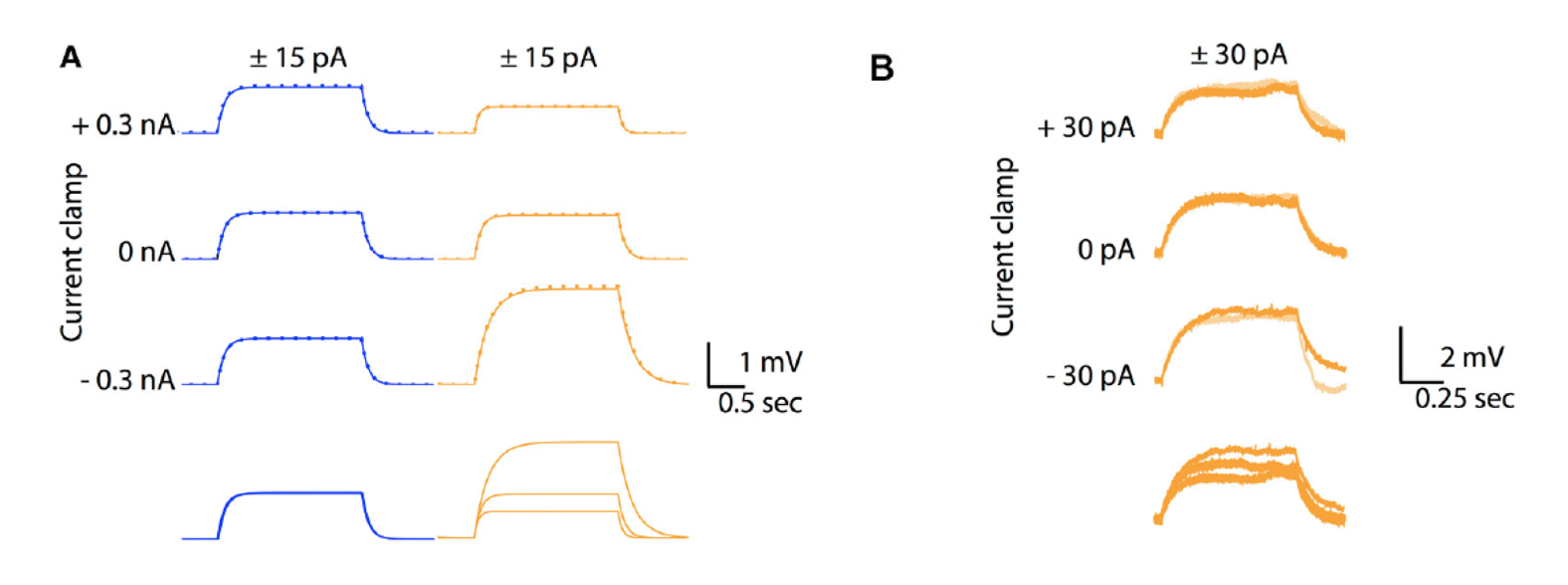
Figure 1: A. Comparison between voltage responses of a passive Ohmic model (blue traces) and a GHK model (orange traces) to to +15 pA (solid lines) and −15 pA (inversed and superimposed, dotted lines) current injections for 2 seconds, at three different holding currents (causing different holding membrane potentials). Note identical responses for the Ohmic model but different sized responses for the GHK model, nevertheless responses to positive and negative currents overlap for both conditions. B. Similar protocol in a Purkinje cell in slice, with all major ionic currents blocked, shows also different sized responses for each holding potential (−74, −67, and −63 mV for bottom to top)
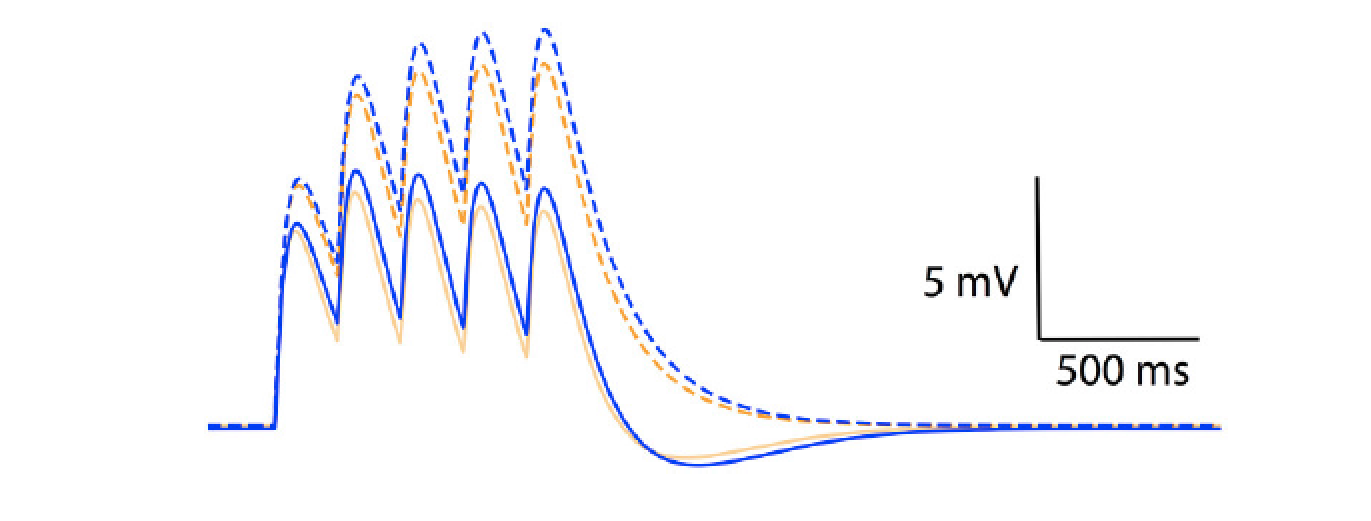
Figure 2: temporal summation of excitatory post-synaptic potentials in an Ohmic model (blue) and a GHK model (orange). Solid and dotted lines represent summation in the presence and absence of Ih respectively. The maximal conductance of Ih used in this simulation is identical to GLeak of the GHK leak current.Content
Model simulations showed that the non-linear leak affects voltage-clamp recordings. The amplitude and rates of transient and steady state voltage properties of the mammalian voltage-gated potassium current Kv4 differed substantially between the Ohmic and GHK model simulations. The underlying cause of this difference was the large K+ gradient in combination with the greater K+ permeability relative to that of Cl−. In contrast, in the squid axon, the K+ gradient is less steep and more importantly, the permeability ratio of K+/Cl− is less than 1, therefore there is little difference between the Ohmic and GHK squid axon models. The non-linear leak also reduces temporal summation of excitatory synaptic input significantly (Figure 2).
Together, our results demonstrate the importance of trans-membrane ionic concentration in defining the functional properties of the passive membrane in mammalian neurons as well as other excitable cells.
Duration of Purkinje cell complex spikes increases with their firing frequency
Climbing fiber triggered complex spikes are massive depolarization bursts of the cerebellar Purkinje cell, showing several high frequency spikelet components (±600 Hz). Since its early observations, the complex spike is known to vary in shape. We analyzed complex spike waveforms, extracellularly recorded in awake primates performing saccades (Warnaar et al., 2015). Every Purkinje cell analyzed showed a range of complex spike shapes with profoundly different duration and number of spikelets. The initial part of the complex spike was rather constant but the later part differed greatly, with a pronounced jitter of the last spikelets causing a large variation in total complex spike duration.
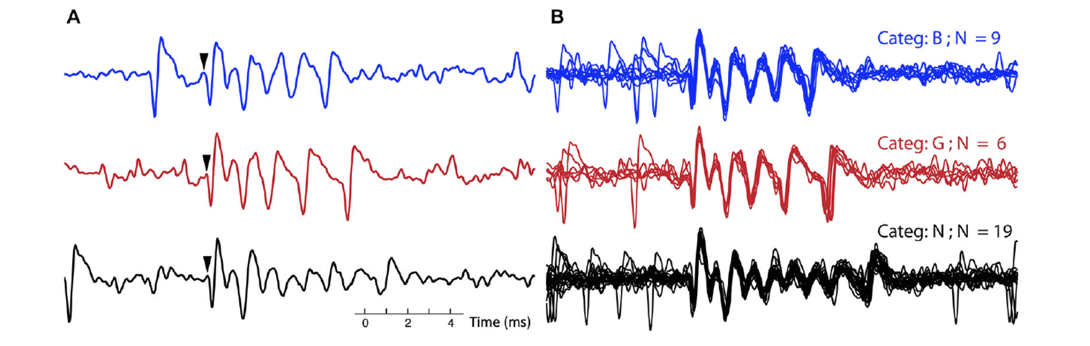
Figure 3: A. Three single complex spikes, each belongs to a different category, in both the blue (top) trace and black (bottom) trace a simple spike precedes the complex spikes. Arrows indicate onset of the complex spike. B. All the complex spikes belonging to each of the three categories, corresponding to the ones in A, are overlaid. The CSs from the three different categories (out of 10 total for this recording) were selected to exemplify clear differences between categories: in number of spikelets (categories B and G versus N) and in timing of final spikelets (category B versus G).
Waveforms did not effect the following pause duration in the simple spike train, nor were simple spike firing rates predictive of the waveform shapes or vice versa. The waveforms did not differ between experimental conditions nor was there a preferred sequential order of complex spike shapes throughout the recordings. Instead, part of their variability, the timing jitter of the complex spike's last spikelets, strongly correlated with interval length to the preceding complex spike: shorter complex spike intervals resulted in later appearance of the last spikelets in the complex spike burst, and vice versa. As a result, the total duration of the complex spike increased with complex spike firing frequency.
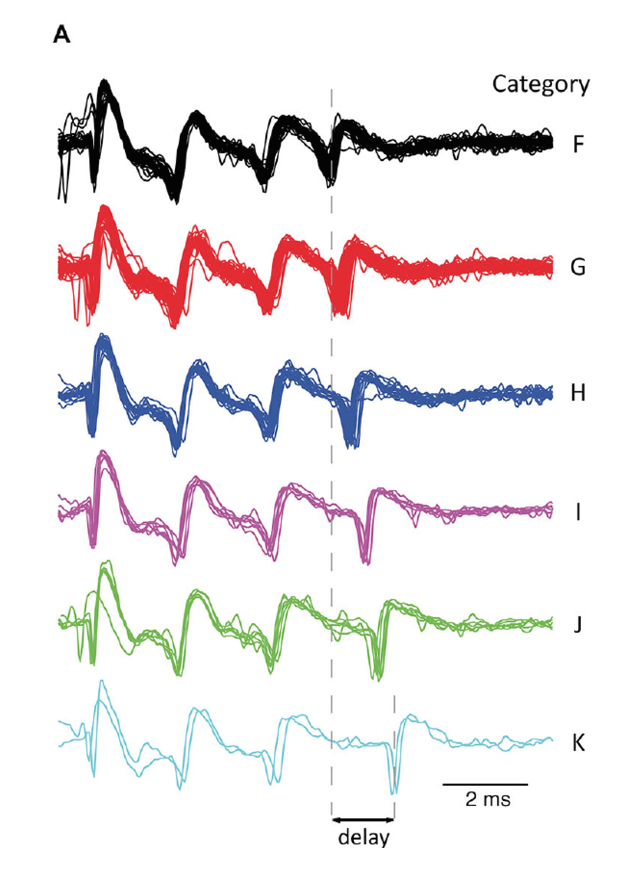
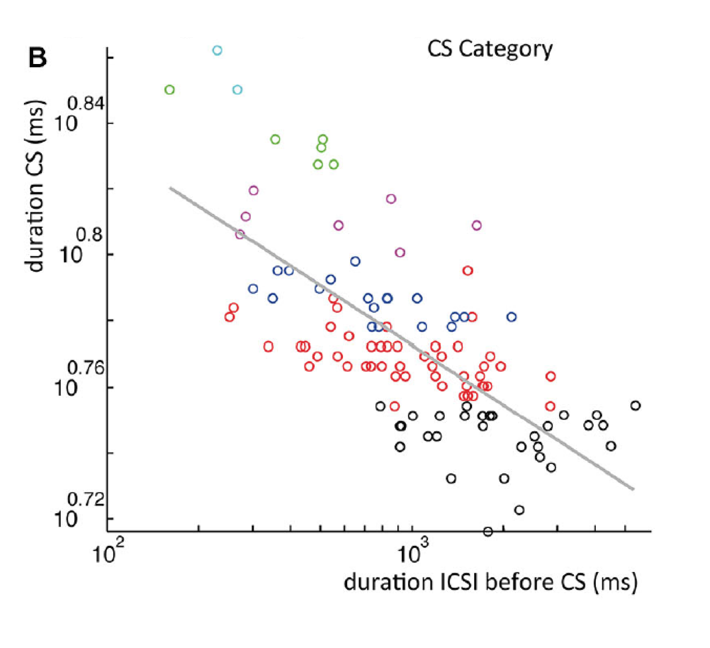
Figure 4: A. All complex spike waveforms that differed only in delay of last spikelet from a single recording. B. Scatterplot of the complex spike duration (time between first and last spikelet) versus interval with preceding complex spike in a log-log plot. Gray line is a power-function fit (power = −0.026) to the data with R2 = 0.49 (p < 0.001). Colors used indicate categories.
A similar phenomenon was observed in rat Purkinje cells recorded in vitro upon repeated extracellular stimulation of climbing fibers at different frequencies in slice experiments. All together these results strongly suggest that the variability in the timing of the last spikelet is due to complex spike frequency dependent changes in Purkinje cell excitability.
Information processing in the olivocerebellar system
Cerebellar nuclear neurons use time and rate coding to transmit Purkinje neuron pauses
Neurons of the cerebellar nuclei convey the final output of the cerebellum to their targets in various parts of the brain. Within the cerebellum their direct upstream connections originate from inhibitory Purkinje cells. The simple firing pattern of Purkinje cells consists of periods of regular fast spiking interrupted by intermittent pauses of variable length and the pauses can be synchronized among Purkinje cells while the regular spikes are not. How can the cerebellar nucleus process this complex input pattern? In a modeling study, we investigated different forms of Purkinje cell simple spike pause synchrony and its influence on candidate coding strategies in the cerebellar nuclei (Sudhakar et al., 2015). That is, we investigated how different alignments of synchronous pauses in synthetic Purkinje neuron spike trains affect either time-locking or rate-changes in the downstream nuclei.
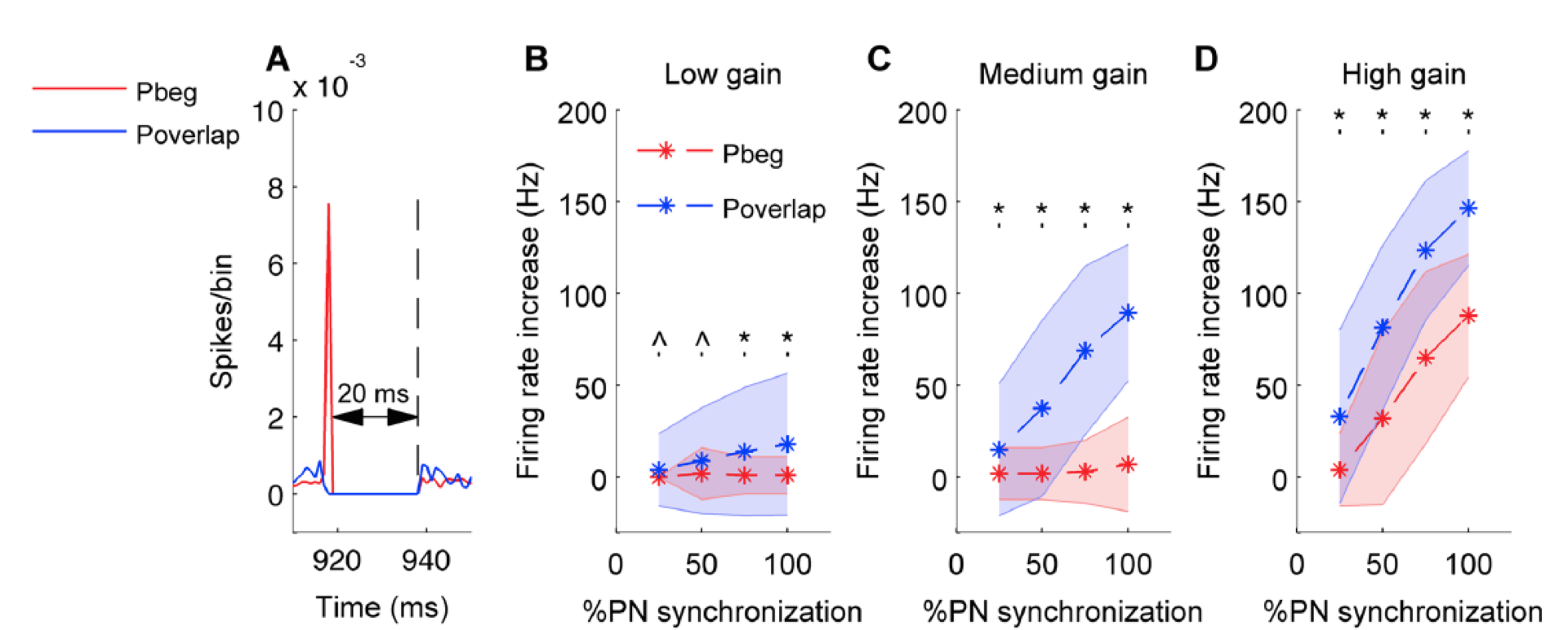
Figure 5: In all panels red color stands for pause beginning condition, blue for pause overlapping condition. Error bars are represented by shaded region. A. Population spike timing histogram of all Purkinje cells projecting onto the cerebellar nucleus neuron model. One can notice synchronous pause of 20 ms for both types of synchronization. B. Mean and standard deviation of increase in firing rate of cerebellar nucleus neuron quantified for both conditions and for low input gain. C. Same for medium input gain. D. Same for high input gain. (*) represents comparisons that are significant (p<0.05) and (^) represents insignificant comparisons (p> = 0.05).
We found that Purkinje neuron synchrony is mainly represented by changes in the firing rate of cerebellar nuclei neurons. Pause beginning synchronization produced a unique effect on nuclei neuron firing, while the effect of pause ending and pause overlapping synchronization could not be distinguished from each other. Pause overlapping synchronization mediated greater firing rate increases than pause beginning synchronization (Figure 5). Pause beginning synchronization produced better time-locking of nuclear neurons for short length pauses (Figure 6).
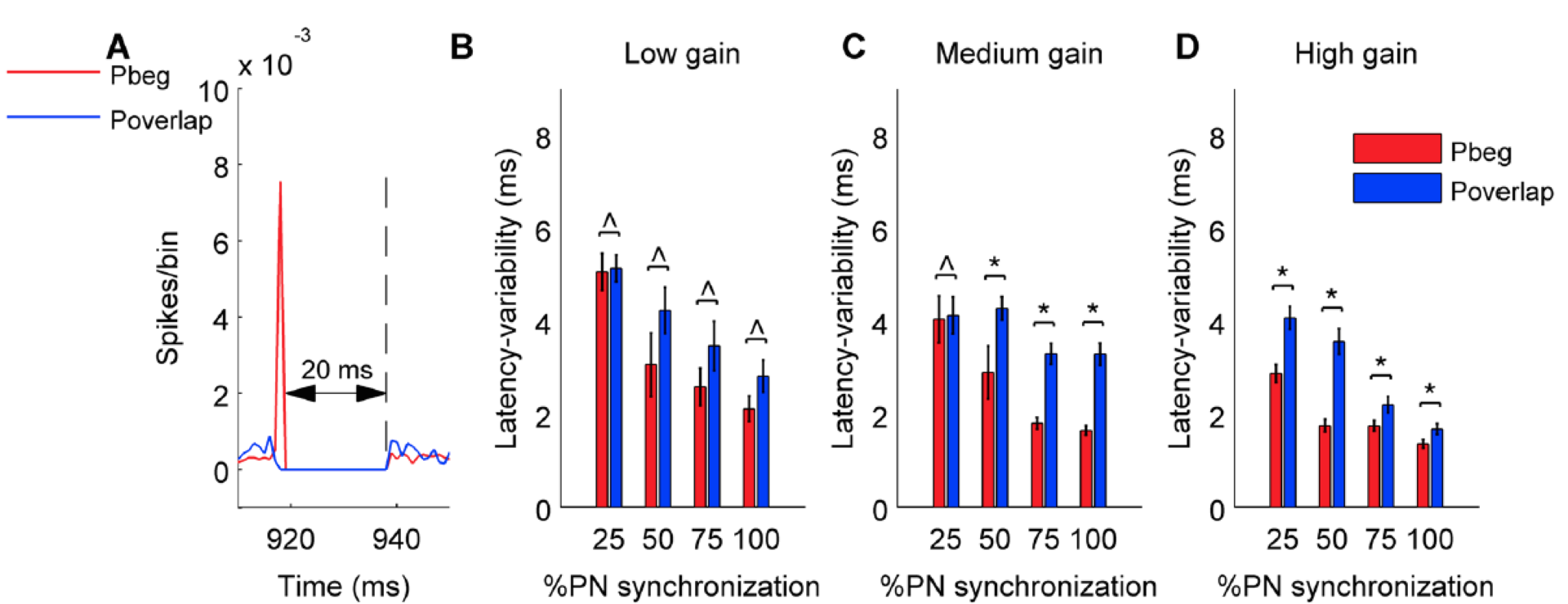
Figure 6: Variability of latency of first cerebellar nucleus neuron spike during the pause calculated from 100 trials for pause beginning and pause overlapping type synchronization. Same panel organization and conventions as Figure 5.
We also characterized the effect of pause length and spike jitter on the nuclear neuron firing. Finally, we demonstrate that the rate of rebound responses in nuclear neurons after a synchronous pause is controlled by the preceding firing rate of its afferent Purkinje cells.
GABA-mediated repulsive coupling between circadian clock neurons in the SCN
The mammalian suprachiasmatic nucleus (SCN) forms not only the master circadian clock but also a seasonal clock. This neural network of ∼10,000 circadian oscillators encodes season-dependent day-length changes through a largely unknown mechanism. We show that region-intrinsic changes in the suprachiasmatic nucleus fine-tune the degree of network synchrony and reorganize the phase relationship among circadian oscillators to represent day length (Myung et al., 2015). Our collaborators measured oscillations of the clock gene Bmal1, at single-cell and regional levels in cultured suprachiasmatic nuclei explanted from animals raised under short or long days. We estimated coupling between ventral and dorsal SCN using the Kuramoto framework showing that the data best matched a network that has couplings that can be both phase-attractive (synchronizing) and -repulsive (desynchronizing) (Figure 7).
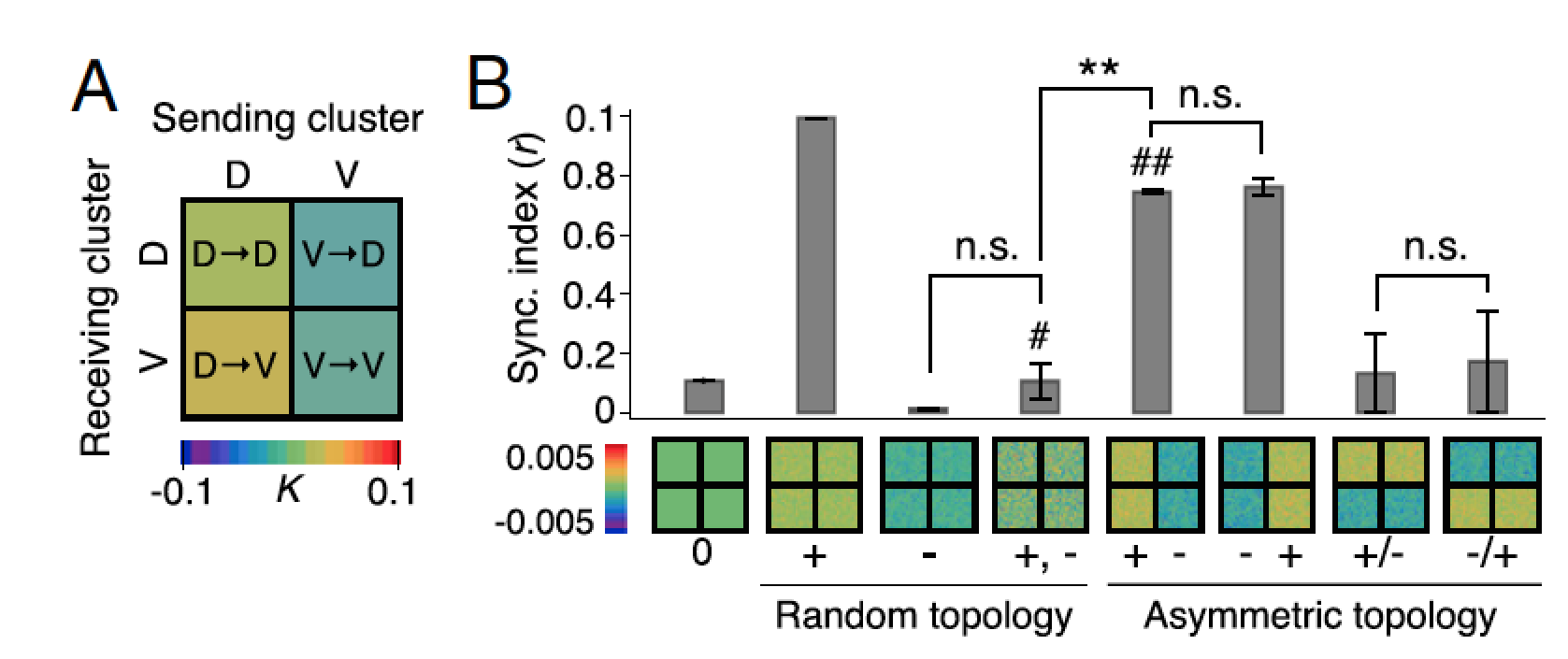
Figure 7: Dynamics of suprachiasmatic nucleus oscillators imply asymmetrically distributed repulsive and attractive phase couplings. A. The phase-model–based estimation of coupling finds a network motif of phase-repulsive coupling (blue) from V- to D-SCN and phase-attractive coupling (red) from D- to V-SCN in the cross-sample mean (SEM ≤0.002, n = 6 12:12 SCNs). B. Surrogate models under various coupling schemes show that partial synchronization emerges under the asymmetric attractive (+)–repulsive (−) coupling motif (marked with ##) found in A, whereas a randomly distributed, structureless (marked with #) 50:50 mixture of attractive and repulsive couplings caused desynchronization (**P < 0.001, Student’s t test for triplicates).
The phase gap between the dorsal and ventral regions increases and the overall period of the SCN shortens with longer day length and the network model replicated these findings (Figure 8D).
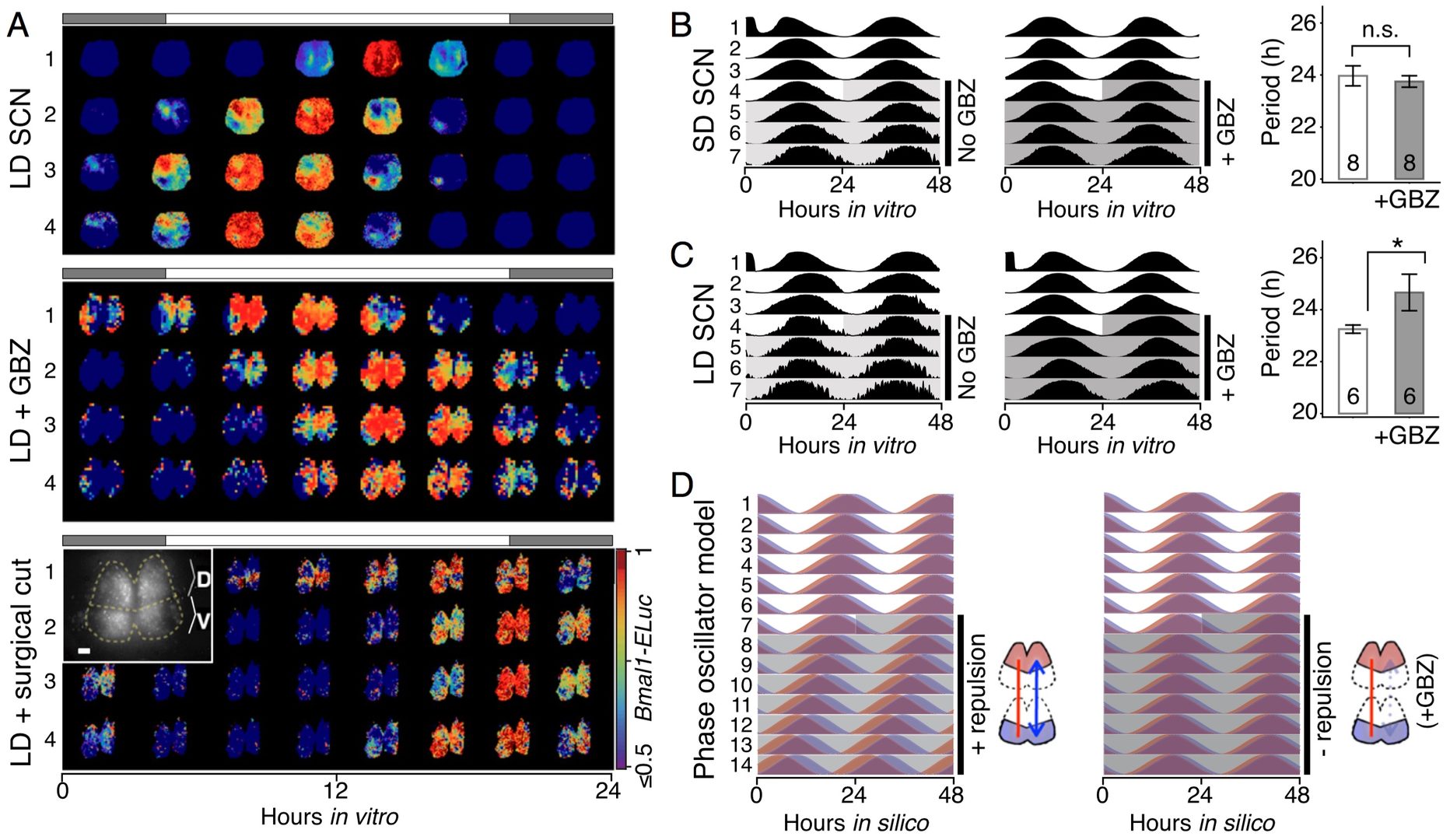
Figure 8: GABAA inhibition disturbs the phase and period organization caused by long or short day length. A. Time-lapse images of normalized Bmal1-ELuc oscillations in the LD-entrained SCNs are shown for every 3 h, color-coded to indicate the peak expression with dark red and lower-than-50% expression with dark blue (color bar on the bottom right). The clear D-V cluster formation and the short oscillation period can be seen in the control LD-entrained SCN (Upper). Application of the GABAA blocker gabazine (GBZ) lengthens the free-running period in culture while making the cluster separation less clear (Middle). Similarly, physical separation by a surgical cut (Inset) lengthens the free-running period in the LD SCN (Lower). (Scale bar, 100 μm.) B. and C. The effect on the free-running period is observed consistently in a larger number of samples through whole-field luminometry using a photomultiplier tube. GBZ has no effect on the SD SCN culture B but in the LD SCN culture C GBZ increases the period (*P < 0.05, Student’s t test; n indicated in the bar graph). D. The asymmetric repulsive coupling model can explain the phase and period organization under LD entrainment. With increased repulsive coupling in the D-SCN, the phase gap between D- and V- SCNs increases and the free-running period becomes shorter, as seen in the LD SCN. The light or dark shade indicates the region of vehicle or drug application.
One of the underlying physiological mechanisms is the modulation of the intracellular chloride concentration, which can adjust the strength and polarity of the ionotropic GABAA-mediated synaptic input. Increasing day-length changes the pattern of chloride transporter expression, yielding more excitatory GABA synaptic input, and that blocking GABAA signaling or the chloride transporter disrupts the unique phase and period organization induced by the day length. These results indicate that the network encoding of seasonal time is controlled by modulation of intracellular chloride, which determines the phase relationship among and period difference between the dorsal and ventral suprachiasmatic nucleus.
4. Publications
4.1 Journals
- De Schutter, E. The Missing Piece of the Puzzle: Neuroinformatics at the Bench. Neuroinformatics 13, 131-132, doi:Doi 10.1007/S12021-015-9268-3 (2015).
- De Schutter, E. Neuroinformatics for Degenerate Brains. Neuroinformatics 14, 1-3, doi:10.1007/s12021-015-9294-1 (2016).
- Gallimore, A. R. Restructuring consciousness - the psychedelic state in light of integrated information theory. Frontiers in Human Neuroscience 9, doi:10.3389/fnhum.2015.00346 (2015).
- Gallimore, A. R., Aricescu, A. R., Yuzaki, M. & Calinescu, R. A Computational Model for the AMPA Receptor Phosphorylation Master Switch Regulating Cerebellar Long-Term Depression. PLoS Comput Biol 12, e1004664, doi:10.1371/journal.pcbi.1004664 (2016).
- Huang, S., Hong, S. & De Schutter, E. Non-linear leak currents affect mammalian neuron physiology. Front Cell Neurosci 9, 432, doi:10.3389/fncel.2015.00432 (2015).
- Myung, J., Hong, S., DeWoskin, D., De Schutter, E., Forger, D. B. & Takumi, T. GABA-mediated repulsive coupling between circadian clock neurons in the SCN encodes seasonal time. Proceedings of the National Academy of Sciences of the United States of America, doi:10.1073/pnas.1421200112 (2015).
- Sudhakar, S. K., Torben-Nielsen, B. & De Schutter, E. Cerebellar nuclear neurons use time and rate coding to transmit Purkinje neuron pauses. PLoS Computational Biology, doi:10.1371/journal.pcbi.1004641 (2015).
- Warnaar, P., Couto, J., Negrello, M., Junker, M., Smilgin, A., Ignashchenkova, A., Giugliano, M., Thier, P. & De Schutter, E. Duration of Purkinje cell complex spikes increases with their firing frequency. Front Cell Neurosci 9, doi:Artn 122, Doi 10.3389/Fncel.2015.001 (2015).
- Wybo, W. A., Boccalini, D., Torben-Nielsen, B. & Gewaltig, M. O. A Sparse Reformulation of the Green's Function Formalism Allows Efficient Simulations of Morphological Neuron Models. Neural Comput 27, 2587-2622, doi:10.1162/NECO_a_00788 (2015).
- Yalgin, C., Ebrahimi, S., Delandre, C., Yoong, L. F., Akimoto, S., Tran, H., Amikura, R., Spokony, R., Torben-Nielsen, B., White, K. P. & Moore, A. W. Centrosomin represses dendrite branching by orienting microtubule nucleation. Nature Neuroscience 18, 1437-1445, doi:10.1038/nn.4099 (2015).
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
Oral Presentations
- De Schutter, E. Stochastic effects in neurons at molecular and cellular levels, in Modeling the brain: from neurons to integrated systems, Erice, Italy (2015).
- De Schutter, E. Stochastic effects at neuron molecular and cellular levels, in 9th IBRO World Congress of Neuroscience, Rio de Janeiro, Brazil (2015).
- De Schutter, E. Multiplexed Coding by Purkinje Cell Simple Spikes, in Gordon Research Conference Cerebellum, Lewiston, ME, USA (2015).
- Gallimore, A. The Neuropharmacological and Evolutionary Implications of the Astonishing Psychoactive Effects of N,N-Dimethyltryptamine (DMT). in Breaking Convention 2015, London, UK (2015).
- Hong, S. Multiplexed coding by cerebellar Purkinje neurons, in CNS 2015 Meeting, Prague, Czech (2015).
- Torben-Nielsen, B. An HPC approach to generate context-dependent virtual neuronal morphologies, in CNS 2015 Meeting, Prague, Czech (2015).
Poster Presentations
- Chen, W., Hepburn, I. & De Schutter, E. Implementation of Parallel Spatial Stochastic Reaction-Diffusion Simulation in STEPS, in CNS 2015 Meeting, Prague, Czech (2015).
- Hepburn, I., Chen, W. & De Schutter, E. Accurate Approximation to Stochastic Reaction Diffusion on Unstructured Meshes in STEPS, in CNS 2015 Meeting, Prague, Czech (2015).
- Sudhakar, S. K., Torben-Nielsen, B. & De Schutter, E. The effect of synchronized pauses on the coding strategies of cerebellar nuclear neurons: A modeling study, in CNS 2015 Meeting, Prague, Czech (2015).
- Sudhakar, S. K., Torben-Nielsen, B. & De Schutter, E. Cerebellar nuclear neurons use time and rate coding to transmit Purkinje neuron pauses, in Society of Neuroscience 2015, Chicago, USA (2015).
- Torben-Nielsen, B. & De Schutter, E. Purkinje cells: the forest shapes the trees, in CNS 2015 Meeting, Prague, Czech (2015).
- Torben-Nielsen, B. & De Schutter, E. The Purkinje cell forest shapes the trees, in Society of Neuroscience 2015, Chicago, USA (2015).
- Vrieler, N., Torben-Nielsen, B., Negrello, M., De Schutter, E., Yarom, Y. & Uusisaari, M. Y. Dendrite arrangement rather than soma locations define clusters of inferior olive neurons, in Society for Neuroscience 2015, Chicago, USA (2015).
- Zang, Y. & De Schutter, E. The ionic mechanism of the Purkinje cell dendritic spikes generation and propagation: a model exploration, in CNS 2015 Meeting, Prague, Czech (2015).
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 Summer Course
OIST Computational Neuroscience Course 2015
- Date: June 08 - 25, 2015
- Venue: Seaside House, OIST
- Co-organizers: E. De Schutter, K. Doya & J. Wickens, OIST
- Co-sponsors: the European Commission, Seventh Framework Programme via the European project CSNII (FP7-ICT-601167)
- Speakers:
- Netta Cohen (University of Leeds, UK)
- Erik De Schutter (OIST)
- Kenji Doya (OIST)
- Eugene Izhikevich (Brain Corporation, USA)
- Bernd Kuhn (OIST)
- Peter Latham (Gatsby Unit, UCL, UK)
- Yukio Nishimura (National Institute for Physiological Sciences, Japan)
- Steve Prescott (University of Toronto, Canada)
- Jonathan Rubin (McGowan Institute for Regenerative Medicine, USA)
- Jackie Schiller (Technion, Israel)
- Greg Stephens (OIST)
- Taro Toyoizumi (RIKEN BSI, Japan)
- Xiao-Jing Wang (New York University, USA)
- Yoko Yazaki-Sugiyama (OIST)
- Wako Yoshida (NICT, CiNet, Japan)
6.2 Internal Seminar
Title : Understanding how the brain does motor control
- Date: February 12, 2016
- Venue: OIST Campus Lab3, C700
- Speaker : Dr. Sergio Verduzco, OIST Researcher
7. Other
Nothing to report.



