Seminar by Prof. Kyoko Nozaki -"Development of New Catalysts toward Utilization of Renewable Resources"
Date
Location
Description
Speaker:
Professor Kyoko Nozaki
Department of Chemistry and Biotechnology,
The University of Tokyo
………………………………………………………
Date: Friday, February 19, 2016
Time: 10:30am – 11:30am
Venue: C700, Lab 3, Level C
………………………………………………………
Title:
"Development of New Catalysts toward Utilization of Renewable Resources"
Abstract:
The development of mild methods for the synthesis of bulk chemicals using renewable feedstocks is a critical technological hurdle along the path to a sustainable chemical economy in the future. Carbon dioxide is one of the most attractive renewable C1 resources, which has many practical advantages such as abundance, economic efficiency, and lack of toxicity. The favorable nature as a carbon source is, however, inextricably linked to its inherent inertness. Here we report a new strategy to circumvent thermodynamic and kinetic barriers for copolymerizations of carbon dioxide and olefins by using a meta-stable lactone intermediate, 3-ethylidene-6-vinyltetrahydro-2H-pyran-2-one, which is formed by the palladium-catalyzed condensation of carbon dioxide and 1,3-butadiene. Subsequent free radical polymerization of the lactone intermediate afforded high-molecular-weight polymers with a carbon dioxide content of 33 mol% (29 wt%). 1
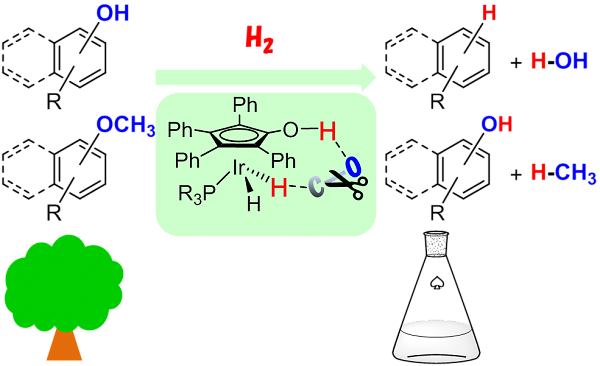
For more than half a century, the chemical industry has mostly depended on petroleum as chemical feedstock. Because oil is hydrocarbon with low oxidation state, various selective oxidation processes have been developed for its conversion to value-added chemicals. Unlike petroleum, renewable resources, plant dry matter for example, is mostly composed of highly oxidized carbon compounds such as cellulose and lignin, which are not very amenable for deoxygenation and subsequent processing into chemical compounds on the market. Thus, the development of an efficient technology involving the chemical reduction becomes a great challenge. Here in this communication, we report direct and selective hydrogenolysis of sp2 C–OH bonds in arenols by hydroxycyclopentadienyl dihydridoiridium catalysts. Hydrogenolysis of sp3 C–O bonds in aryl methyl ethers were also achieved using the same catalysts. These catalyses were further applied to deoxygenation of a lignin model compound, implying the potential application for mass production of arenes from lignin or its degraded components.2
References
1. Nakano, R.; Ito, S.; Nozaki, K. Nature Chem. 2014, 6, 325-331
2. S. Kusumoto, K. Nozaki, Nature Commun. 2015, 6, 6296.
Attachments
Subscribe to the OIST Calendar: Right-click to download, then open in your calendar application.



