FY2018 Annual Report
Coordination Chemistry and Catalysis Unit
Assistant Professor Julia Khusnutdinova

Abstract
During FY2018, we investigated in detail the model systems for photoluminescent Cu(I) complexes previously used by our group as the dynamic probes for detection and visualization of mechanical stress in polymer samples. Our model studies provide deeper understanding of the correlation between dynamic properties of conformationally flexible ligands in these probes and their photophysical properties.
We also studied two extreme cases of "truly innocent" and "doubly non-innocent" ligands in Ni and Mn complexes, respectively. In these studies, we further challenge the concept of metal-ligand cooperation and ligand's participation in bond activation by designing and studying two types of ligands: one leading to observation of unusual double dearomatization of the pyridine rings in the ligand, and another where any ligand's participation is fully blocked leading to observation of unusual oxidation states at the metal.
1. Staff
- Dr. Orestes Rivada Wheelaghan, Postdoctoral Scholar
- Dr. Pradnya Patil, Postdoctoral Scholar
- Dr. Ayumu Karimata, Postdoctoral Scholar
- Dr. Abir Sarbajna, Postdoctoral Scholar
- Dr. Olga Gladkovskaya, Research Unit Technician
- Sebastien Lapointe, Graduate Student
- Shubham Deolka, Graduate Student
- Hoan Dinh, Graduate Student (Laboratory Rotation)
- Manuel Angel Gallardo Villagran, Research Intern
- Luke Kramer, Research Intern
- Jiratheep Pruchyathamkorn, Research Intern
- Saiyyna Stepanova, Research Intern
- Alèria Garcia Roca, Research Intern
- Kyoko Chinen, Research Unit Administrator
2. Collaborations
2.1 Electron rich, bulky PNP ligands
- Synthesis and reactivity studies of complexes with electron-rich, bulky PNP ligands
- Type of collaboration: Joint research
- Researchers:
- Dr. Eugene Khaskin (STG, OIST)
2.2 CO2 Reduction Catalyzed by Earth-Abundant Metal Complexes
- Description: Utilization of manganese complexes with proton-responsive ligands for reduction of CO2 to value-added products.
- Type of collaboration: Joint research
- Researchers:
- Professor Carlo Nervi, University of Turin, Italy
3. Activities and Findings
3.1 Dynamics and brightness: interplay between conformational dynamics and photophysical properties of Cu(I) complexes
In 2017, we first reported conformationally flexible Cu(I) complexes incorporated into polyurethanes as mechanophores allowing detection and visualization of mechanical stress. When polyurethane samples containing Cu-based mechanophores were stretched, increase of the emission intensity was observed. The unique feature of this system was fast and reversible response, unlike in many existing systems where irreversible changes or slow recovery were seen. This is due to a different mechanism of mechanoresponse that does not involve scission or formation of covalent chemical bonds, which usually requires significant structural reorganization and intrinscically slow or irreversible changes. We hypothesized that in our Cu-based mechanophores, changes in photoluminescence intensity in response to stress are due to subtle changes in coordination environment of a Cu(I) center and the rigidity of the macrocyclic ligand attached to a polymer chain. To further study with idea, we prepared a series of model compounds in which we utilized sterics of the ligand to slow down conformational dynamics of the macrocyclic ligand framework. We observed good correlation between conformational flexibility and photoluminescence quantum yield, consistent with our previous hypothesis that emission intensity changes in polymer-incorporated systems are due to suppressing dynamics of the complex.
In 2018, we reported our detailed investigation of the macrocyclic ligand conformational behavior in solution, solid-state structures and the photophysical properties of copper(I) cationic and neutral mononuclear complexes supported by tetradentate N,N′-dialkyl-2,11-diaza[3.3](2,6)-pyridinophane ligands RN4 (R = H, Me, iBu, secBu, neoPent, iPr, Ts). Steric properties of the alkyl group at the axial amine in the RN4 ligand were found to strongly affect the conformational preferences and dynamic behavior in solution. Several types of conformational exchange processes were revealed by variable-temperature NMR and 2D exchange spectroscopy, including degenerative exchange in a pseudotetrahedral species as well as exchange between two isomers with different conformers of tri- and tetracoordinate RN4 ligands. These exchange processes are slower for the complexes containing bulky alkyl groups at the amine compared to less sterically demanding analogues. A clear correlation is also observed between the steric bulk of the alkyl substituents and the photoluminescent properties of the derived complexes, with less dynamic complexes bearing bulkier alkyl substituents exhibiting higher absolute photoluminescence quantum yield (PLQY) in solution and the solid state: PLQY in solution increases in the order Me < neoPent < iBu < secBu ≈ iPr < tBu. The electrochemical properties of the cationic complexes [(RN4)CuI(MeCN)]X (X = BF4, PF6) were also dependent on the steric properties of the amine substituent.
This work was supported by JSPS KAKENHI Grant JP18K05247
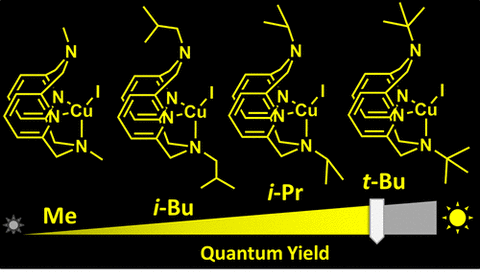
Figure 1: Copper(I) complexes containing alkyl substituents with variable steric bulk used in this study.
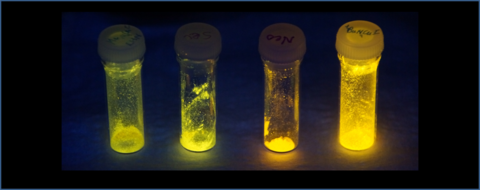
Figure 2: Photoluminescent Cu(I) complexes under UV light irradiation. The most bright complex on the right corresponds to the most bulky complex with t-Bu substituent, while the least bright compound on the left contains the least bulky complex with Me substituent.
3.2 "Doubly guilty": extending the scope of ligand non-innocence to Mn complexes with N,S-donor ligands
Metal-ligand cooperation is an important tool for activation of strong chemical bonds, in which both the metal and the ligand participate in the reactivity, impyting the ligand itself is "non-innocent" during the bond activation process. In particular, in the case of phosphine-substituted pyridine-based pincer complexes, metal-ligand cooperation involves deprotonation on the methylene group attached to a pyridine ring, which assists in heterlytic splitting of H-H, H-O, H-N and other bonds. However, phosphine ligands are typically characterized by low stability in air and they are also synthetically challenging and expensive. For practical application, replacement of phosphines with sulfur-based fragments would be highly advantageous due to their higher stability to air and lower cost. However, metal-ligand cooperation in sulfur-containing pyridine-based ligands has been complicated by irreversible undesirable side-reactions such as defragmentation of the sulfur-containing fragment, or by unfavorable stability of the resulting species.
In this work, we take advantage of the macrocyclic structure of the sulfur-containing pyridinophane ligand N2S2 to stabilize reactive deprotonated ligands attached to a manganese center. Manganese complexes currently are actively investigated as active catalysts for (de)hydrogenation and other bond activation processes, in which ligand's non-innocence has been proposed. In this work, we were able to observe for the first time single and double dearomatization of pyridine rings in MnI complexes with an N2S2 pyridinophane ligand via deprotonation of one or two CH2 arms, respectively. In contrast to other N,S-donor pincer-like systems, the dearomatized (N2S2)Mn species were found to be stable, with the dearomatization being reversible.Double dearomatization of the pyridine rings potentially opens up new possibility for bond activation, which will be the focus of our next study.
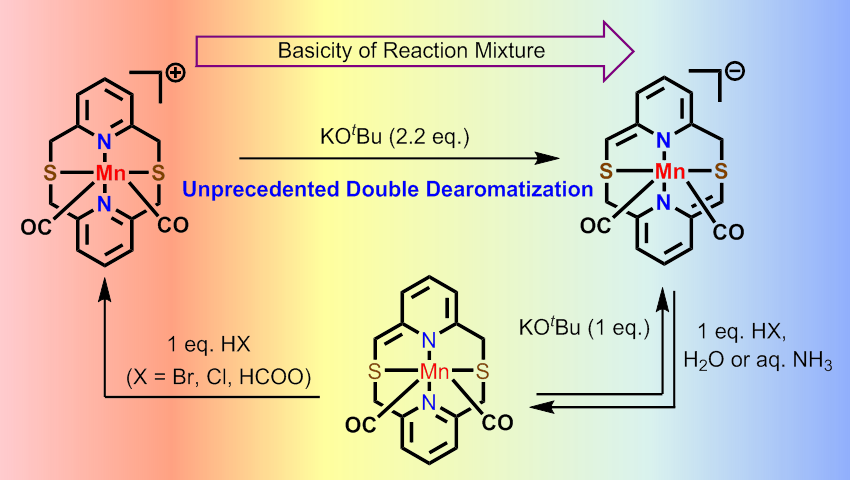
Figure 3: Single and double dearomatization of pyridine rings in N2S2 ligand in Mn(I) complexes.
3.3 "Truly innocent": disabling ligand-based reactivity in new electron rich, sterically hindered pincer ligands leads to stabilization of unusual Ni(I) complexes
As explained in the section above, metal-ligand cooperation is an important concent in chemistry of pyridine-based phosphine-containing pincer ligands (PNP ligands). While it has been instrumental in studying bond activation, it typically obscures a redox-reactivity that would otherwise occur at the metal center. At the same time, observing metal-based rather than ligand-based reactivity is improtant for understanging of the reactivity of the metal center in unusual oxidation states, and in particular, in studying paramagnetic organometallic compounds, their structure and reactivity, which might lead to discovery of new types of bond activation processes. In this work, we set out to investigate the reactivity of the Ni complexes with a newly designed Me4PNP ligand, in which we block any possible ligand-based reactivity by substitution the CH2 positions with four Me groups, thus making a truly innocent PNP ligand. Moreover, these newly synthesized ligands also demonstrate high electron-donating properties and increased steric hindrance at the metal center. Interestingly, instead of a ligand-based reactivity reported in previous works on (PNP)Ni complexes, we observed stabilization of unusual Ni(I) oxidation state in the resulting complexes. Moreover, we found that steric hindrance at the phosphine ligand plays an important role in determining geometry of the metal center. Complexes containing iPr groups on the phosphorus atoms show a rare seesaw geometry around the metal center, while tBu-substituted complexes show a distorted square-planar geometry. Computational analysis reveals the SOMO for all complexes has a dx2-y2 character with the spin density mostly residing on the nickel.
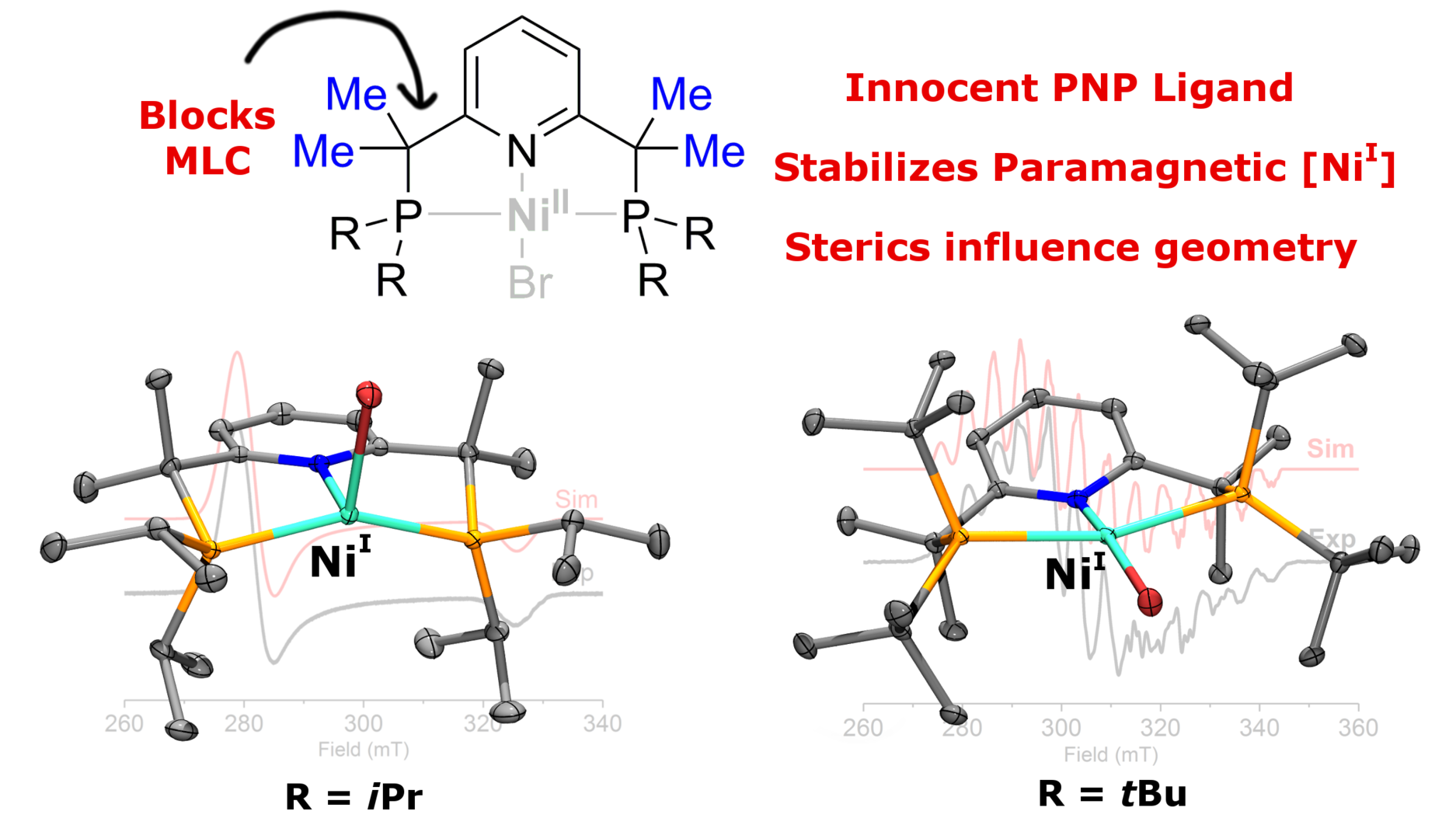
Figure 4: Ni(I) complexes stabilized by tetramethyl-substituted PNP ligands.
4. Publications
4.1 Journals
- Patil, P. H.; Filonenko, G. A.; Lapointe, S.; Fayzullin, R. R.; Khusnutdinova, J. R. "Interplay between the Conformational Flexibility and Photoluminescent Properties of Mononuclear Pyridinophanecopper(I) Complexes." Inorg. Chem. 57, 10009-10027, doi:10.1021/acs.inorgchem.8b01181 (2018).
-
Sarbajna, A.; Patil, P. H.; Dinh, M. H.; Gladkovskaya, O.; Fayzullin, R. R.; Lapointe, S.; Khaskin, E.; Khusnutdinova, J. R. "Facile and reversible double dearomatization of pyridines in non-phosphine MnI complexes with N,S-donor pyridinophane ligand" Chem. Comm. 2019, DOI: 10.1039/C9CC00424F.
- Lapointe, S.; Khaskin, E.; Fayzullin, R. R.; Khusnutdinova, J. R. "Stable Nickel(I) Complexes with New Electron-Rich, Sterically-Hindered, Innocent PNP Pincer Ligands" Organometallics 2019, ASAP, DOI 10.1021/acs.organomet.9b00026.
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Patil, P. H. & Khusnutdinova, J. R. In Influence of Tetradentate Macroclic Ligand Sterics on the Conformational Flexibility and Photoluminescent Properties of Copper(I) Complexes., in 255th American Chemical Society National Meeting, New Orleans, USA, 2018.03.18.
- Khusnutdinova, J. R., Karimata, A. & Patil, P. H. Stimuli-responsive polymers through dynamic coordination compounds. ICCC, Sendai, Japan, 2018.08.01.
- Rivada-Wheelaghan, O.; Khusnutdinova, J. R. Unsymmetrical naphthyridinone-based ligand scaffolds for the development of linear chain multi-metallic complexes, ICCC, Sendai, Japan, 2018.08.01.
- Sarbajna, A.; Dubey, A.; Khusnutdinova, J. R. Ligand-assisted H2 activation by manganese complexes, ICCC, Sendai, Japan, 2018.08.02.
- Khusnutdinova, J. R. Dynamic coordination compounds of CuI: new mechanophores and extended metal atom chains, invited talk at the 25th International SPACC Symposium, Okinawa, Japan, 2018.11.23.
- Lapointe, S. & Khaskin, E., Khusnutdinova, J. R. Stable Ni(I) Complexes With New Electron-Rich, Sterically Hindered PNP Pincer Ligands, in The 99th Annual Meeting 2019 of the Chemical Society of Japan, Kobe, Japan, 2019.3.17.
- Deolka, S. & Khaskin, E. Unusual Rearrangement of a Modified PNP Ligand based Ru Complexes. , in The 99th Annual Meeting 2019 of the Chemical Society of Japan, Kobe, Japan, 2019.03.17.
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 Seminars Hosted
1. "Catalytic hydrogenation and dehydrogenative coupling." (hosted by Science and Technology Group)
- Date: May 7, 2018
- Venue: OIST Campus Center Building (C210)
- Speaker: Prof. Dmitry G. Gusev (Wilfrid Laurier University)
2. "Catalytic Transformations of Phosphaalkynes and Alkylated Dihydropyridines"
- Date: May 30, 2018
- Venue: OIST Campus Lab 3 (C700)
- Speaker: Dr. Kazunari Nakajima (The University of Tokyo)
3. "Catalyst Design with Solid-supported Ligands and Metals"
- Date: August 21, 2018
- Venue: OIST Campus Lab 1 (B503)
- Speaker: Prof. Masaya Sawamura (Hokkaido University)
4. "Metal-Metal and Metal-Ligand Cooperation for Activating Small Molecules: Unprecedented Rate Acceleration for Oxygen Atom Transfer"
- Date: September 13, 2018
- Venue: OIST Campus Center Building (C210)
- Speaker: Dr. Graham de Ruiter (Technion)
5. "Design of Mechanochromic Polymers Based on Dynamic Covalent Chemistry"
- Date: October 29, 2018
- Venue: OIST Campus Lab 3 (C700)
- Speaker: Prof. Hideyuki Otsuka (Tokyo Institute of Technology)
6. "Metal–Ligand Cooperation in Protic Pyrazole and N-Heterocyclic Carbene Complexes"
- Date: November 13, 2018
- Venue: OIST Campus Lab 1 (B503)
- Speaker: Prof. Shigeki Kuwata (Tokyo Institute of Technology)
7. Other
Internal Seminar: Lapointe, S., Khaskin, E., & Khusnutdinova, J. R. "Novel Bulky PNP Pincer Ligands: the Effect on the Stabilization and Geometry of Unusual Oxidation States of Ni Complexes". April 13, 2018.
CSJ Presentation Award 2018 to presentation 4A7-11 at the 98th CSJ meeting: Rivada-Wheelaghan, O. & Khusnutdinova, J. Unsymmetrical Naphthyridinone-based Ligand Scaffolds for a Controlled and Reversible Stepwise Growth of Linear Chain Multi-metallic Complexes (2018.03.23)
CSJ Student Presentation Award 2019 to presentation 2C1-01 at the 99th CSJ meeting: Lapointe, S. & Khaskin, E., Khusnutdinova, J. R. Stable Ni(I) Complexes With New Electron-Rich, Sterically Hindered PNP Pincer Ligands (2019.3.17).



