Developmental Neurobiology Unit (Ichiro Masai)
Unit outline

Research
Abstract
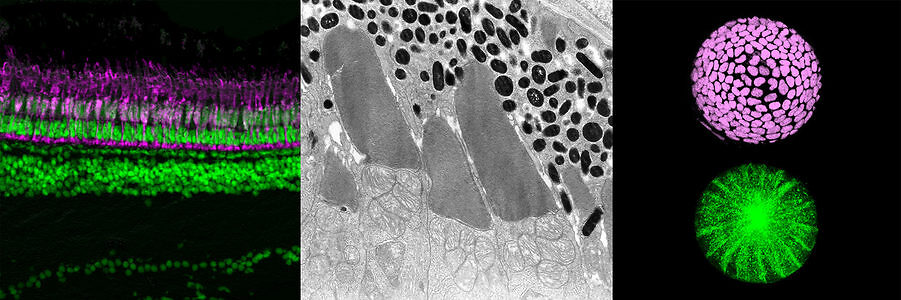
Vertebrate retina is derived from the ventral region of forebrain. In this region, six major classes of retinal neurons differentiate and form neural circuit responsible for vision. Thus, the retina provides a good model for studying cell differentiation and neural circuit formation. Using zebrafish as an animal model, we have investigated following topics:
- Mechanisms that regulate retinal neurogenesis and neural circuit formation
- Mechanisms that regulate retinal cell death including photoreceptor degeneration
- Mechanisms that regulate lens fiber differentiation
From 1998 to 2004, we performed large-scale mutagenesis using zebrafish, and isolated more than 300 zebrafish mutants showing defects in retinal and lens development. These mutant defects are classified into different categories: neurogenesis, neuronal differentiation, neural circuit formation, retinal cell death, including photoreceptor degeneration, and lens fiber differentiation. We initially focused on mechanisms that regulate retinal neurogenesis. From 2004, we started to study mechanisms underlying retinal cell death, including photoreceptor degeneration. From 2006, when my lab moved from RIKEN to OIST, we started to investigate mechanisms that regulate lens fiber differentiation. Throughout these research projects, we have established concepts that govern development of multicellular organisms and contribute to understanding pathological processes involved in human eye diseases.
References
Chiang, H.-J., Nishiwaki, Y., Chiang, W.-C., and Masai, I. (2024) Male germ cell-associated kinase is required for axoneme formation during ciliogenesis in zebrafish photoreceptors. Dis. Model Mech. dmm.050618. doi: 10.1242/dmm.050618.
Sugiyama, Y., Reed, D., Herrmann, D., Lovicu, F., Robinson, M.L., Timpson, P., and Masai, I. (2024) Fibroblast Growth Factor-induced lens fiber elongation is driven by Rho and Rac. Development 151, dev.202123. doi:10.1242/dev.202123.
Babu, S., Takeuchi, Y. and Masai, I. (2022) Banp regulates DNA damage response and chromosome segregation during the cell cycle in zebrafish retina. eLife. 11:e74611. doi: 10.7554/eLife.74611.
Ahmed, M, Kojima, Y., and Masai, I. (2022) Strip1 regulates retinal ganglion cell survival by suppressing Jun-mediated apoptosis to promote neural curcuit formation. eLife. 11:e74650. doi:10.7554/eLife.74650.
Ranawat, N., and Masai, I. (2021) Mechanism underlying microglial colonization of developing neural retina in zebrafish. eLife. 10:e70550. doi:10.7554/eLife.70550.
Nishiwaki, Y., and Masai, I. (2020) β-SNAP activity in the outer segment growth period is critical for preventing BNip1-dependent apoptosis in zebrafish photoreceptors. Sci. Rep. 10: 17379.
Iribarne, M., Hyde, D. R. and Masai, I. (2019) TNFα induces Müller glia to transition from non-proliferative gliosis to a regenerative response in mutant zebrafish presenting chronic photoreceptor degeneration. Front. Cell Dev. Biol. 7, 296. DOI:10.3389/fcell.2019.00296
Mochizuki, T., Kojima, Y., Nishiwaki, Y., Harakuni, T., and Masai, I. (2018) Endocytic trafficking factor VPS45 is essential for spatial regulation of lens fiber differentiation in zebrafish. Development, 145, pii dev170282.
Iribarne, M. Nishiwaki, Y., Nakamura, S., Araragi, M., Oguri, E. and Masai, I. (2017) Aipl1 is required for cone photoreceptor function and survival through the stability of Pde6c and Gc3 in zebrafish. Sci. Rep. 7:45962.
Mochizuki, T., Luo, Y. J. Tsai, H. F. Hagiwara, A., and Masai, I. (2017) Cell division and cadherin-mediated adhesion regulates lens epithelial cell movement in zebrafish. Development 144, 708 – 719.
Mochizuki, T., Suzuki, S., and Masai, I. (2014) Spatial pattern of cell geometry and cell-division orientation in zebrafish lens epithelium. Biol. Open 3, 982–994.
Imai, F., Yoshizawa, A., Matsuzaki, A., Oguri, E., Araragi, M., Nishiwaki, Y. & Masai, I. (2014) Stem-loop binding protein is required for retinal cell proliferation, neurogenesis, and intraretinal axon pathfinding in zebrafish. Dev. Biol. 391, 94-109.
Mochizuki, T. & Masai, I. (2014) The lens equator: a platform for molecular machinery that regulates the switch from cell proliferation to differentiation in the vertebrate lens. Dev. Growth Differ. 56, 387-401.
Nishiwaki, Y., Yoshizawa, A., Kojima, Y., Oguri, E., Nakamura, S., Suzuki, S., Yuasa-Kawada, J., Kinoshita-Kawada, M., Mochizuki, T., and Masai, I. (2013) The BH3-only SNARE BNip1 mediates photoreceptor apoptosis in response to vesicular fusion defects. Dev. Cell 25, 374 – 387.
Imai, F., Yoshizama, A., Fujimori-Tonou, N., Masai, I. (2010) The ubiquitin proteasome system is required for cell-cycle progression of the lens epithelium and differentiation of lens fiber cells in zebrafish. Development 137, 3257-3268.
Yamaguchi M, Imai F, Tonou-Fujimori N, Masai I. (2010) Mutations in N-cadherin and a Stardust homolog, Nagie oko, affect cell-cycle exit in zebrafish retina. Mech. Dev. 127, 247-264.
Yamaguchi M, Fujimori-Tonou N, Yoshimura Y, Kishi T, Okamoto H, Masai I. (2008) Mutation of DNA primase causes extensive apoptosis of retinal neurons through the activation of DNA damage checkpoint and tumor suppressor p53. Development 135, 1247-1257.
Nishiwaki Y, Komori A, Sagara H, Suzuki E, Manabe T, Hosoya T, Nojima Y, Wada H, Tanaka H, Okamoto H, Masai I. (2008) Mutation of cGMP phosphodiesterase 6alpha'-subunit gene causes progressive degeneration of cone photoreceptors in zebrafish. Mech. Dev. 125, 932-946.
Yamaguchi M, Tonou-Fujimori N, Komori A, Maeda R, Nojima Y, Li H, Okamoto H, Masai I. (2005). Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development 132, 3027-3043.
Masai I, Yamaguchi M, Tonou-Fujimori N, Komori A, Okamoto H. (2005) The hedgehog-PKA pathway regulates two distinct steps of the differentiation of retinal ganglion cells: the cell-cycle exit of retinoblasts and their neuronal maturation. Development 132, 1539-1553.
Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, Wada H, Tanaka H, Nojima Y, Hammerschmidt M, Wilson SW, Okamoto H. (2003) N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development 130, 2479-2494.
Masai I, Stemple DL, Okamoto H, Wilson SW. (2000) Midline signals regulate retinal neurogenesis in zebrafish. Neuron 27, 251-263.