"Polyelectrolytes with hydrophobic counterions: from fundamental physics to hand sanitisers"Carlos Gonzalez Lopez
Date
Location
Description
Micro/Bio/Nanofluidics (Shen) Unit would like to invite you to the seminar by Dr. Carlos Gonzalez Lopez on December 1st (Thursday).
----------------------------------------------------------------------
Date: Thursday, December 1, 2022
Time: 11:00-12:00
Venue: L4E01 (Lab 4)
----------------------------------------------------------------------
Speaker:
Dr. Carlos Gonzalez Lopez
Institute of Physical Chemistry,
RWTH Aachen University, Germany
Title:
Polyelectrolytes with hydrophobic counterions: from fundamental physics to hand sanitisers
Abstract:
Classical models of polyelectrolyte solutions treat ions as point-like charges and neglect counterion-solvent interactions. This picture has been challenged by an array of theoretical considerations and simulation work in recent years. Experimental work on this topic remains relatively sparse in part because of the limited solubility of polyelectrolytes in non-aqueous media, which severely limits the number and type of solvents which can be studied. Here we overcome this limitation by studying polyelectrolytes with large hydrophobic counterions, which display broad solubility in organic media.
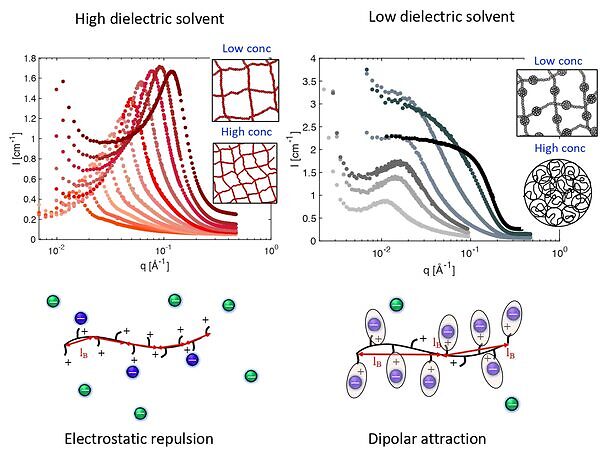
A study of polyelectrolytes in a wide range of organic solvents reveals that as the solvent permittivity decreases, the charge density on the chain decreases, leading to partial or full collapse of polyelectrolyte chains into pearl-necklace or globular structures. Surprisingly, it is found that polyelectrolyte collapse occurs both under solvophobic and solvophilic conditions. The driving force for collapse under solvophilic conditions appears to be the formation of dipoles along the chain when counterions condense onto the backbone. In low dielectric media (ε ⪝ 25), dipolar attraction overcomes electrostatic repulsion between dissociated charges. This crossover is reminiscent of a polyelectrolyte-to-ionomer transition.
Polyelectrolytes with hydrophobic ions have potential uses in hand sanitisers. These typically consist of 80wt% ethanol or isopropanol mixed in water, with 1wt% added polymer to viscosify the mixture. Most commercial polyelectrolytes cannot be used for this purpose due to their insolubility in alcohol media. We show that replacing alkaline counterions by tetra-alkyl-ammonium counterions makes many synthetic or biological polyelectrolytes soluble in alcohols, paving their way for their use in hand sanitisers.
Host:
Prof. Amy Shen
Subscribe to the OIST Calendar: Right-click to download, then open in your calendar application.



