FY2015 Annual Report
Micro/Bio/Nanofluidics Unit
Professor Amy Shen

Abstract
Since moving to our the lab space in Lab 3 in June 2015, Micro/Bio/Nanofluidics Unit (MBNU) has been busy setting up the lab and acquiring key instruments to conduct research in micro-nano-biofluidics and rheology. We now have the full capacity in micro- and nano-fabrications of 2D and 3D devices and structures, in high resolution and high speed flow visualizations in microfluidic devices, and full/advanced rheological characterization tools.
In research, our unit focused on two main thrusts. One is related to the fundamental aspects of hydrodynamics, flow instability, and mixing behavior in various microfluidic platforms (i.e., cross-slot, T-shape, flow focusing), with both Newtonian and non-Newtonian fluids. Another thrust is to use micro- and nanofluidics for biotechnology applications, with emphasis on cell encapsulation, particle/cell separation, and bio-nanomaterials synthesis. For example, micro and nano-contact printing has been integrated in microfluidic devices for multiplexed immunoassay reactions. We also initiated new research directions in biosensing, taking advantage of novel nanomaterials and new sensing techniques. Members of the MBNU unit published 11 papers in FY2015, with our research highlighted in BBC news, R&D Magazine, Science Daily and such.
The unit members have also actively disseminated our research results to the general public and scientific commmunities. We have given a total of 28 presentations and seminars in FY2015, while participating core professional society meetings such as the Society of Rheology Annual Meeting, micro-TAS, IEEE-Nanomed meeting, ASME global conference on Nanoengineering for medicine and biology. Our unit sponsored activity "WALKING ON WATER" was a huge success at the annual open campus event at OIST.
Our unit organized 2 successful OIST mini-symposiums, one titled "Small Meets Large: Connecting Microfluidics with Marine Ecology", another titled "Current Trends and Emerging Technologies in Nanofluidics and Nanofabrications". In addition, the MBNU Unit also hosted 13 internationally renowned scientists visiting OIST and giving research seminars in FY 2015, with topics ranging from microfluidics, microneedles, hydrodynamics of bacteria and biofilms.
Personnel wise, we have been actively recruiting new members. We welcomed 3 new Ph.D students, Mr. Hsieh-Fu (Paul) Tsai, Ms. Shivani Sathish, and Ms. Noa Burshtein in FY 2015. The unit recruited 2 new postdocs, Dr. Francesco Del Giudice from University of Naples Federico II in Italy, and Dr. Nikhil Bhalla from University of Bath in UK. Our unit also welcomed a JSPS fellow, Dr. Vivianne Lutz Bueno from ETH Zurich, who spent 4 months in our lab to pursue collaborative project on wormlike micellar solutions in microfluidics. Former postdoc, Dr. Sebastien Ricoult, took a great position at Illumina London in February 2016. Our group is truly international and diverse, as we come from the US, UK, Italy, France, Austria, India, Israel, Korea, Japan, Taiwan, and Australia.
1. Group Member
As of March 31, 2016
- Prof. Amy Shen, Professor
- Dr. Simon Haward, Group Leader
- Dr. Doojin Lee, Postdoctoral Scholar
- Dr. Casey James Galvin, Postdoctoral Scholar
- Dr. Francesco Del Giudice, Postdoctoral Scholar
- Dr. Nikhil Bhalla, Postdoctoral Scholar
- Mr. Kazumi Toda-Peters, Technician
- Mr. Alexander Unterkreuter, Technician
- Mr. Hsieh-Fu Tsai, Graduate Student
- Ms. Shivani Sathish, Graduate Student
- Ms. Noa Burshtein, Graduate Student
- Mr. Tsung-Han Hsieh, Graduate Student (Rotation)
- Mr. Sergey Zobnin, Graduate Student (Rotation)
- Mr. Rintaro Hayashi, Research Intern
- Ms. Shwetha Meena Sakthi Nallasivam, Research Intern
- Ms. Vivianne Lutz Bueno, Reserach Intern
- Mr. Arifur Rahman, Research Intern
- Ms. Yuno Kaneshi, Research Administrator
Alumni
- Dr. Michael William Boehm, Staff Scientist (Now teaching in Shanghai)
- Dr. Sébastien Ricoult, Postdoctoral Scholar (Now working at Illumina London)
- Ms. Christina Helen Ripken, Research Intern (Now a Ph.D student at OIST)
- Ms. Ya Zhao, Research Intern (Just received her Ph.D at U. of Washington, US)
- Mr. Masashi Kaneda, Research Intern (Back to Hokkaido University)
- Mr. Aniket Ravan, Research Intern (Now a Ph.D student at UIUC, US)
2. Collaborations
2.1 Interfacial dynamics and instability in microfluidics
- Type of collaboration: Joint research
- Researchers:
- Professor Gerry Fuller, Stanford University
- Professor Gareth McKinley, MIT
- Dr. Simon Haward, OIST
- Ms. Noa Burshtein, OIST
2.2 Scattering and dynamics of complex fluids in microfluidics
- Type of collaboration: Joint research
- Researchers:
- Professor Peter Fischer, ETH Zurich
- Dr. Simon Haward, OIST
2.3 Micro and Nanofluidics for Biotechnology applications
- Type of collaboration: Joint research
- Researchers:
- Professor Tadashi Yamamoto, OIST
- Professor Ulf Skoglund, OIST
- Mr. Paul Tsai, OIST
3. Activities and Findings
3.1 Microfluidics and Rheology (fundamental aspects)
3.1.1 Tricritical Spiral Vortex Instability in Cross-Slot Flow
S.J. Haward, R.J. Poole, M.A. Alves, P.J. Oliveira, N. Goldenfeld, and A.Q. Shen, Physical Review E 93, 031101 (2016) – Rapid Communication
We examine fluid flow through cross-slot devices with various depth:width ratios α. At low Reynolds number, Re, flow is symmetric and a sharp boundary exists between incoming fluid streams. Above an α-dependent critical value Rec(α), a steady symmetry-breaking bifurcation occurs and a spiral vortex structure develops. Order parameters characterizing the instability grow according to a 6th order Landau potential, and show a progression from 2nd to 1st order transitions as α increases beyond α = 0.55. Flow simulations indicate the instability is driven by vortex stretching at the stagnation point.
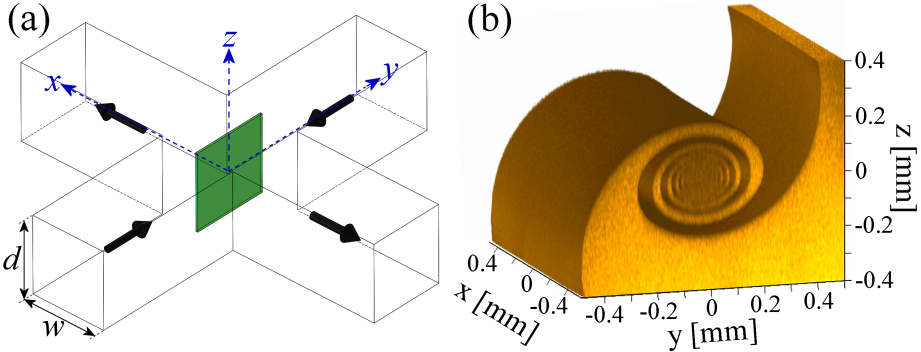
(a) Schematic diagram of a cross-slot device. Fluorescently-dyed fluid enters from positive y and undyed fluid enters from negative y. Flow exits along the x-direction. Confocal microscopy is performed in z-planes, which are scanned through the full depth of the device and used to reconstruct images in the x = 0 plane (green shaded region). (b) Three-dimensional (3D) rendering of a vortex structure observed for the flow of water at Re = 75.8 in a cross-slot with α = 1. The image is generated from z-plane images spaced at δz = 5 μm and has been cropped around the central vortex. The volume shown corresponds to the fluorescently-dyed fluid stream.
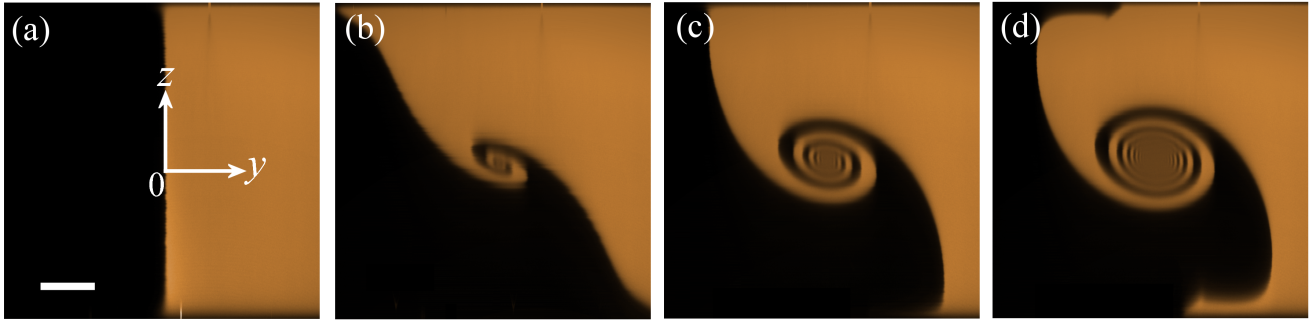
Development of the spiral vortex structure in the x = 0 plane as the Reynolds number (Re) is varied: (a) Re = 15.2, (b) Re = 42.8, (c) Re = 60.6, (d) Re = 91.0. Scale bar in (a) represents 200 µm.
This work has now been taken up by Noa Burshtein as a Ph.D. project and is being extended to quantitatively examine the flow velocity fields and vortex dynamics and to probe the effects of fluid elasticity on the onset and nature of the flow transitions. We anticipate a subsequent paper about vortex suppression by polymer additives to be ready for submission by December 2016.
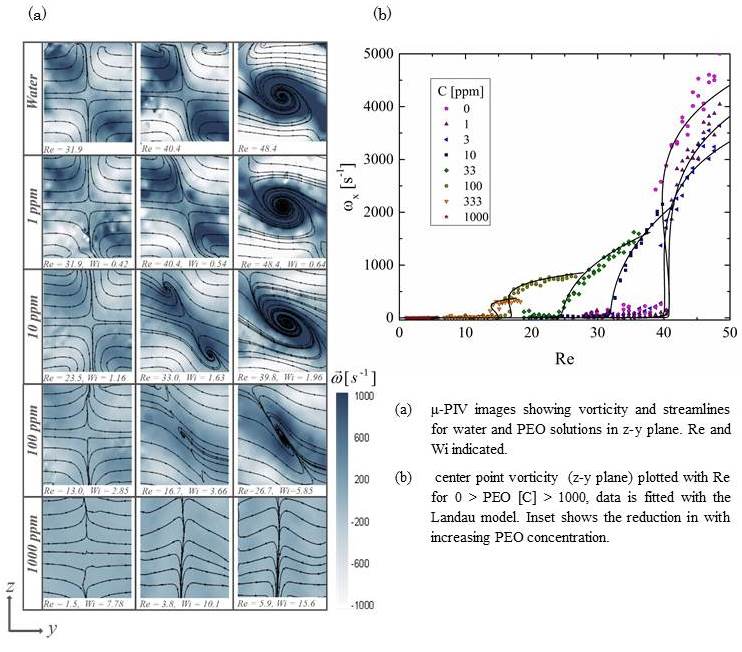
3.1.2 Spiral vortex formation in cross-slot flow
Noa Burshtein, Simon J. Haward, and Amy Q. Shen
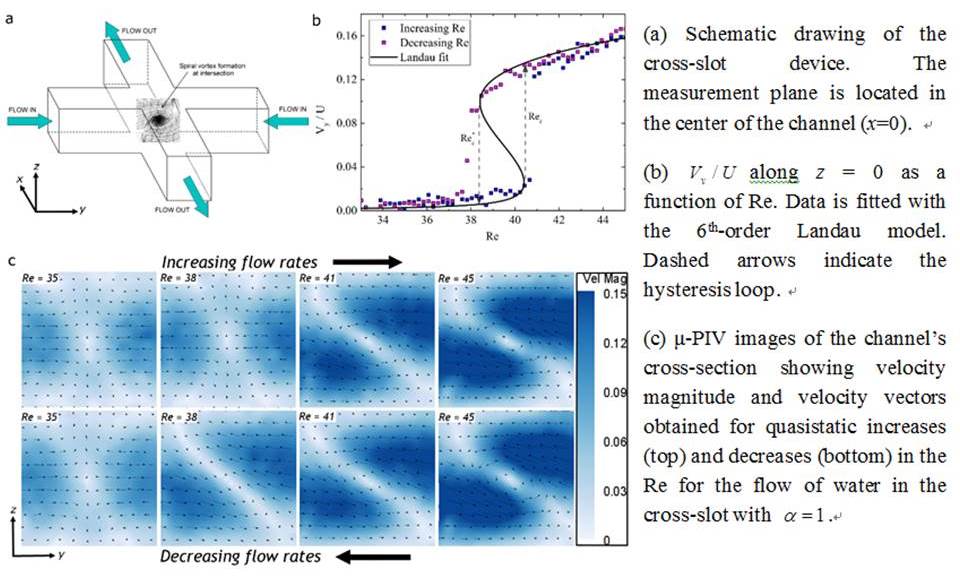
Newtonian flow in cross-slot channels results in the formation of a steady spiral vortex instability as the Reynolds number is increased above a modest critical value (Rec). The value of Rec is strongly dependent on the channel aspect ratio, ɑ=d/w, where d and w are the depth and width of the channel, respectively. This symmetry-breaking flow bifurcation can be described by a Landau-type 6th-order polynomial potential used to characterize tricritical instabilities and phase transitions (Haward et al., Phys. Rev. E 93, 031101, 2016). Here, we employ glass cross-slot devices with ɑ=1, fabricated by selective laser-induced etching (LightFabTM), and we use high speed quantitative flow velocimetry to examine the onset mechanism and the dynamic development of the instability over a range of Re. We experimentally show that for ɑ=1 the transition is 1st order and hysteretic (Figure 1). Our observations support previous numerical simulations showing that the transition results from the growth of center-point vorticity induced by formation of Dean vortices in the channel cross-section. The mechanism of the transition can be described in four stages [1] symmetric flow - centrifugal instability results in the formation of a four-cell pattern of Dean vortices around a central stagnation point; [2] symmetry breaking - one pair of diagonally-opposed Dean cells, with the same rotation direction intensifies at the expense of the second counter-rotating pair; [3] vortices merging – the two intensifying vortices approach each other and ultimately combine on the flow axis; [4] steady central vortex – the transition is complete,
3.1.3 Effect of fluid elasticity on spiral vortex formation in cross-slot flow
Noa Burshtein, Simon J. Haward, and Amy Q. Shen
The effects of viscoelasticity on the onset and development of a spiral vortex instability in the cross-slot channel with an aspect ratio α=1 are studied with poly(ethylene oxide) solutions (PEO, molecular weight M = 4 MDa) over a range of concentrations. This flow instability has potential to enhance and control the mixing of complex fluids at the low Re typically encountered in microfluidic devices. Controlling flow stability and mixing in microfluidic channels are two important problems in the design of lab-on-a-chip devices, which often involve flows of non-Newtonian fluids. Here, we use quantitative flow velocimetry measurements to conduct a controlled study of the effects of viscoelasticity on the onset and development of the instability. The Weissenberg and Reynolds number were used to characterize the flow. We find that increasing the concentration of polymer results in an increase in Wic and a reduction of Rec; it also suppresses the intensification of vorticity and distorts the central spiral shape. For both Newtonian and non-Newtonian fluids, the bifurcation can be described with the Landau model of phase transitions.
3.1.4 Elastic Instabilities in Planar Elongational Flow of Monodisperse Polymer Solutions
Simon J. Haward, Gareth H. McKinley, and Amy Q. Shen, Scientific Reports, 6, 33029, 2016.
We investigate purely elastic flow instabilities in the almost ideal planar stagnation point elongational flow field generated by a microfluidic optimized-shape cross-slot extensional rheometer (OSCER). We use time-resolved flow velocimetry and full-field birefringence microscopy to study the flow behavior of a series of well-characterized viscoelastic polymer solutions under conditions of low fluid inertia and over a wide range of imposed deformation rates. At low deformation rates the flow is steady and symmetric and appears Newtonian-like, while at high deformation rates we observe the onset of a flow asymmetry resembling the purely elastic instabilities reported in standard-shaped cross-slot devices. However, for intermediate rates, we observe a new type of elastic instability characterized by a lateral displacement and time-dependent motion of the stagnation point. At the onset of this new instability, we evaluate a well-known dimensionless criterion M that predicts the onset of elastic instabilities based on geometric and rheological scaling parameters. The criterion yields maximum values of M which compare well with critical values of M for the onset of elastic instabilities in viscometric torsional flows. We conclude that the same mechanism of tension along curved streamlines governs the onset of elastic instabilities in both extensional (irrotational) and torsional (rotational) viscoelastic flows.
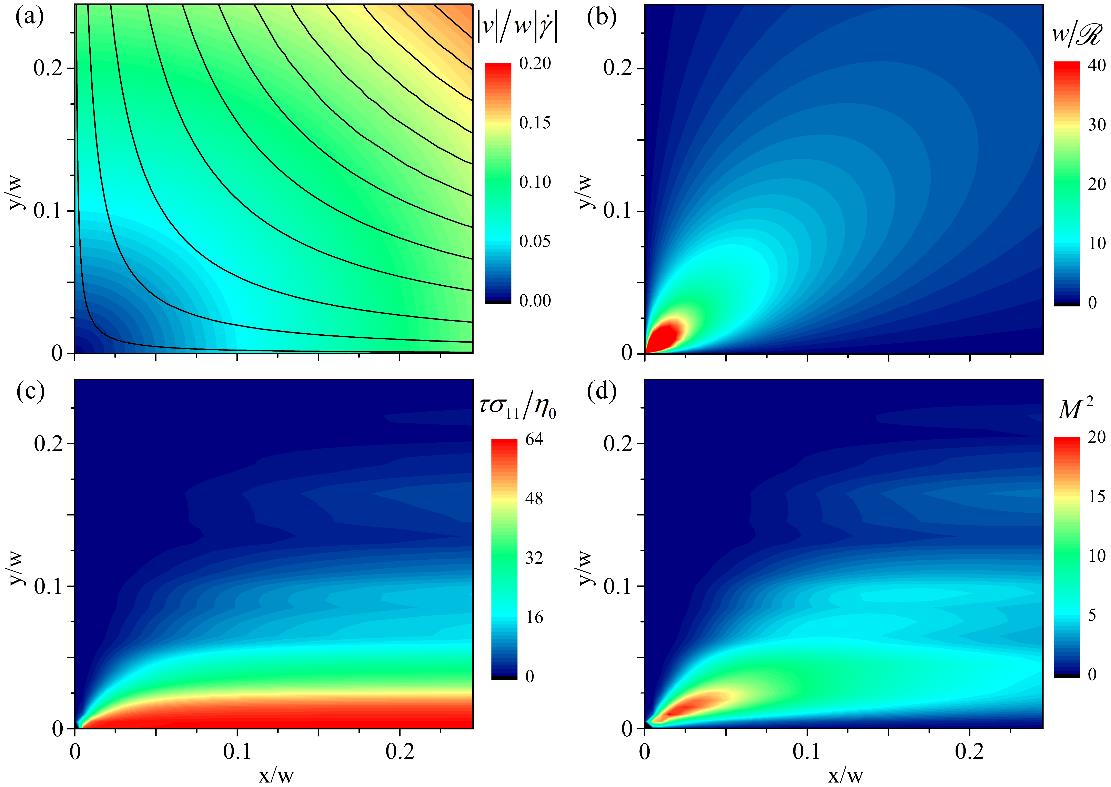
Determination of the elastic instability criterion M2 at the onset of elastic instability for a 0.035 wt.% solution of PS16 in the OSCER device. (a) dimensionless velocity field and hyperbolic streamlines determined using the ideal stream function for planar elongational flow. (b) dimensionless streamline curvature. (c) dimensionless streamwise stress determined from birefringence measurements made at Wi = 0.7, Re = 0.04. (d) Spatial distribution of M2 values.
3.1.5 Flow of Wormlike Micellar Solutions Around Confined Microfluidic Cylinders
Ya Zhao, Amy Q. Shen, Simon J. Haward, DOI: 10.1039/C6SM01597B, 2016.
Wormlike micellar (WLM) solutions are frequently used in enhanced oil and gas recovery applications in porous rock beds where complex microscopic geometries result in mixed flow kinematics with strong shear and extensional components. Experiments with WLM solutions through model microfluidic porous media have revealed a variety of complex flow phenomena, including the formation of stable gel-like structures known as a Flow-Induced Structured Phase (FISP), which undoubtedly play an important role in applications of WLM fluids, but are still poorly understood. A first step in understanding flows of WLM fluids through porous media can be made by examining the flow around a single micro-scale cylinder aligned on the flow axis. Here we study flow behavior of an aqueous WLM solution consisting of cationic surfactant cetyltrimethylammonium bromide (CTAB) and a stable hydrotrope 3-hydroxy naphthalene-2-carboxylate (SHNC) in microfluidic devices with three different cylinder blockage ratios, β. We observe a rich sequence of flow instabilities depending on β as the Weissenberg number (Wi) is increased to large values while the Reynolds number (Re) remains low. Instabilities upstream of the cylinder are associated with high stresses in fluid that accelerates into the narrow gap between the cylinder and the channel wall; vortex growth upstream is reminiscent of that seen in microfluidic contraction geometries. Instability downstream of the cylinder is associated with stresses generated at the trailing stagnation point and the resulting flow modification in the wake, coupled with the onset of time-dependent flow upstream and the asymmetric division of flow around the cylinder.
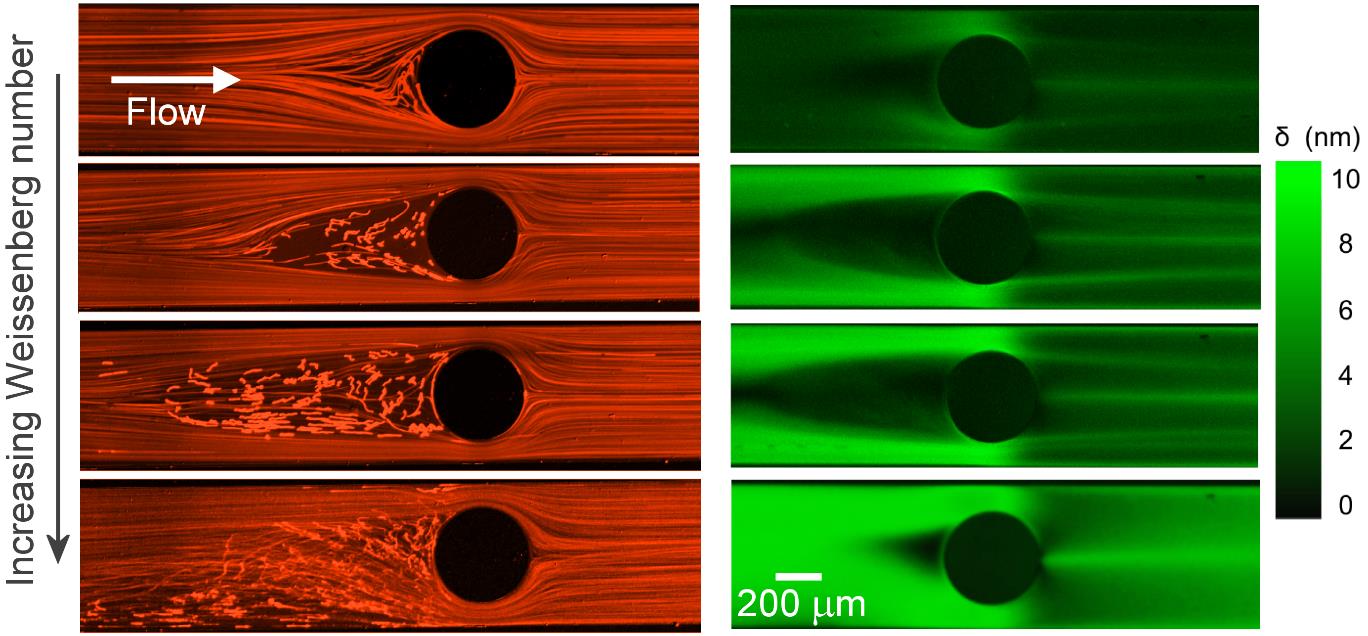
Fluorescent streak imaging (left) and corresponding flow-induced birefringence imaging (right) for flow of a CTAB/SHNC WLM solution past a microfluidic confined cylinder (β = 0.67) as the Weissenberg number is progressively increased from top to bottom.
3.1.6 Integrated microfluidic platform for instantaneous flow and localized temperature control
Cifeng Fang, Doojin Lee, Boris Stober, Gerald G. Fuller, and Amy Q. Shen, RSC Advances, 5, 85620–85629, 2015.
We developed an integrated microfluidic platform for instantaneous flow and localized temperature control. The platform consisted of a flow-focusing region for emulsion droplet production, a cross junction region embedded with a microheater for droplet trapping and localized temperature control by using an active feedback control system. The droplet trapping performance and trapping stability were also investigated. We further used thermotropic liquid crystal as the droplet phase to demonstrate the trapping and temperature control ability of the microfluidic platform. Our integrated platform offers the capability of manipulating non-contact, instantaneous flow with localized temperature control, which provides valuable tools for studying transient interfacial dynamics and various biological and industrial processes.
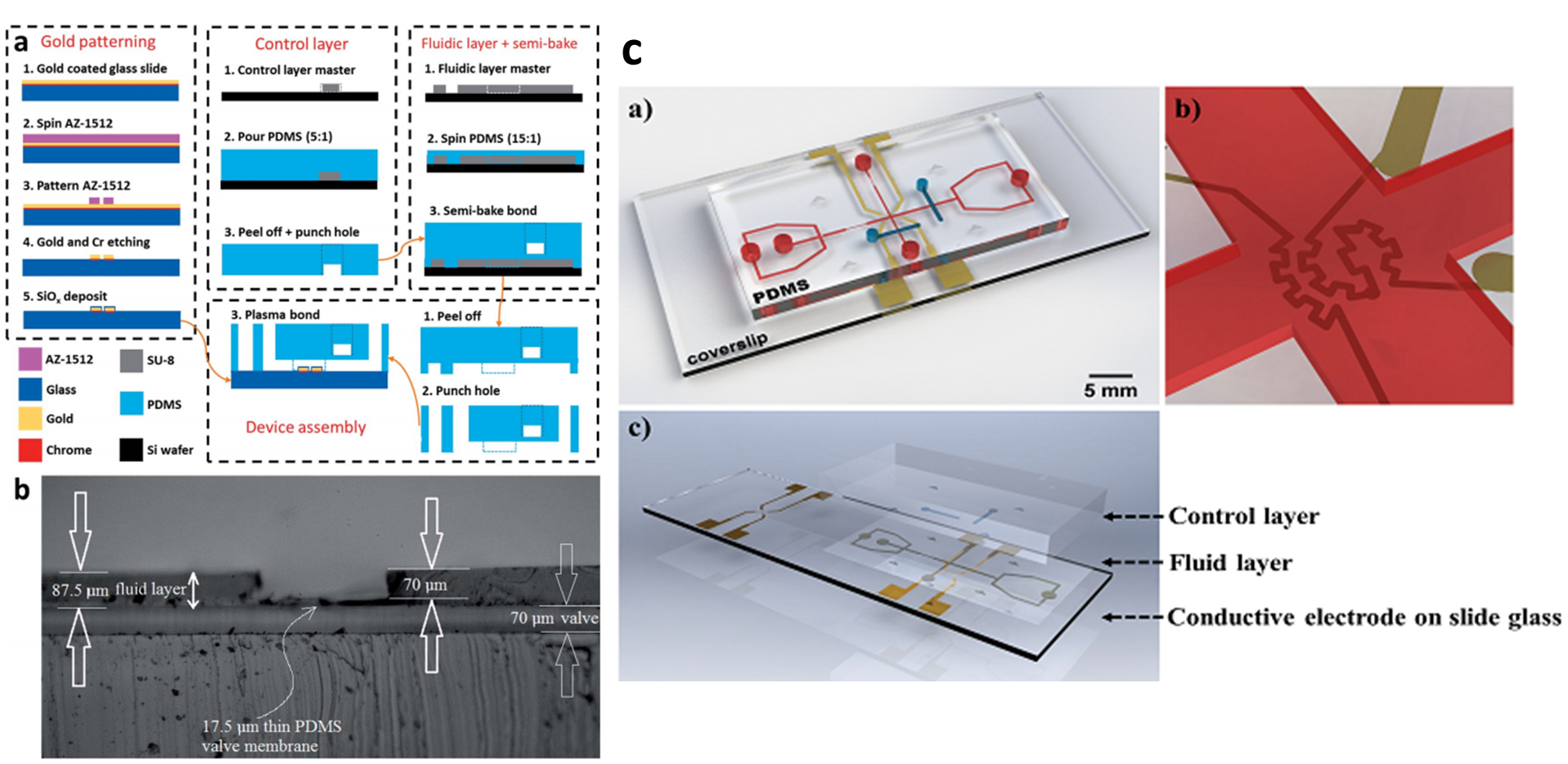
Device fabrication and characterizations: (a) cross-section view highlighting key device features, step-by-step procedure of conductive patterning, double-layered PDMS channel fabrication, and device assembly (b) optical microscopy image of the cross section of the double-layered PDMS device, showing the structure of the membrane valve. (c) Integrated microfluidic platform for flow and temperature control: a) 3D rendering illustration of the device, b) microheater and temperature sensor at the cross-junction, and c) device assembly.
Device fabrication and characterizations: (a) cross-section view highlighting key device features, step-by-step procedure of conductive patterning, double-layered PDMS channel fabrication, and device assembly (b) optical microscopy image of the cross section of the double-layered PDMS device, showing the structure of the membrane valve. (c) Integrated microfluidic platform for flow and temperature control: a) 3D rendering illustration of the device, b) microheater and temperature sensor at the cross-junction, and c) device assembly.
3.1.7 Temperature controllable microfluidic tensiometer
Doojin Lee, Cifeng Fang, Aniket S. Ravan, Gerald G. Fuller, and Amy Q. Shen, to be submitted.
Temperature variations affect the intrinsic properties of fluids significantly. Thus, on-chip temperature control becomes important to obtain stable operations inside microfluidic devices. Conventional temperature control methods either preheat the carrying fluid inside the device or use a printed wiring board heating unit under the entire microfluidic device. It is challenging to obtain precise localized temperature control in a small area inside the microchannel. Here, we developed integrated microfluidic device with temperature control to precisely control the temperature inside the chip. Temperature control is accomplished by employing a custom designed LabVIEW virtual interface that communicates with a gas regulator, temperature controller and a digital multimeter. Our integrated microfluidic platform offers great advantages on providing direct real-time measurement of transient interfacial dynamics which is relevant to numerous technological processes, ranging from cleansing operations where liquid soaps are rinsed from surfaces, to the dissolution of mucous in the intestinal tract.
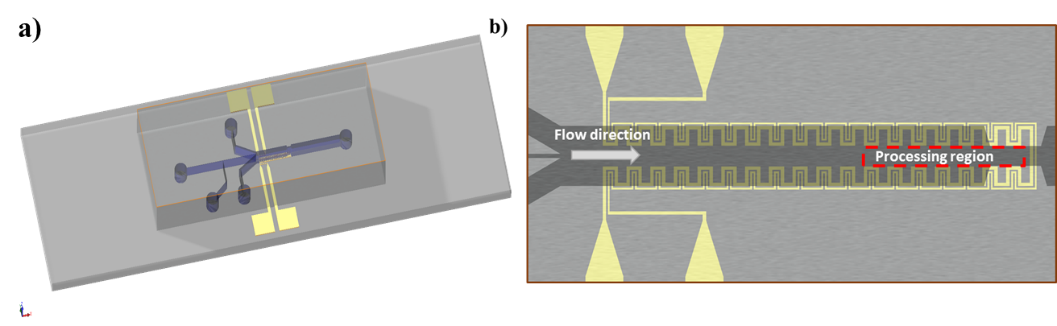
Schematic representation of a microfluidic tensiometer integrated with a temperature controller. a) Isometric view and b) top view of the device.
3.1.8 Relaxation time of dilute polymer solutions: a microfluidic approach
Francesco Del Giudice, Simon J. Haward and Amy Q. Shen (Manuscript in Preparation)
Dilute polymer solutions are very important for the study of many fundamental problems, related to polymer stiffness, molecular conformations, etc. In the recent years, dilute polymer solutions have been widely used for many microfluidic applications. These studies highlight the importance of an accurate measure of the fluid relaxation time. For dilute polymer solutions, it is very challenging to measure the small relaxation time by using conventional techniques. However, microfluidic techniques provide a unique opportunity to meet such challenges. In this work, we compare the relaxation time from shear and extensional experiments, both measured through microfluidic techniques. Relaxation time down to 100 μs are successfully measured. Our findings suggest a strong relation between the measured relaxation time and the external applied flow field. Moreover, we found that at concentration c closed to the overlapping concentration c*, the data are well described by the Rouse model (fully screened hydrodynamic interactions). Fig. 1a shows the relaxation time λ as a function of the concentration ratio c/c* derived through x-slot and μ-rheometer respectively for atactic polystyrene in DOP solutions. The relaxation time shows a dependence from the concentration λ_(x-slot)∝c^0.41 and λ_(μ-rheo)∝c^0.46 even for c⁄c* <1. Fig.1b shows the same comparison as in Fig.1a for the HA solution in PBS and different NaCl concentrations. In this case, data derived from the x-slot experiments agree well with those derived from the μ-rheometer around c/c* =1, i.e, close to the dilute regime. The relaxation time displays a concentration dependence in the dilute polymer regime, with λ_(x-slot)~λ_(μ-rheo)∝c in agreement with theoretical predictions. A constant relaxation time λ≃0.1 ms is experimentally found for c⁄c* <0.1 .
In conclusion, microfluidic approaches enabled us to study dilute polymer solutions down to c⁄c*~0.01, even in the case of aqueous solutions where other techniques fail.
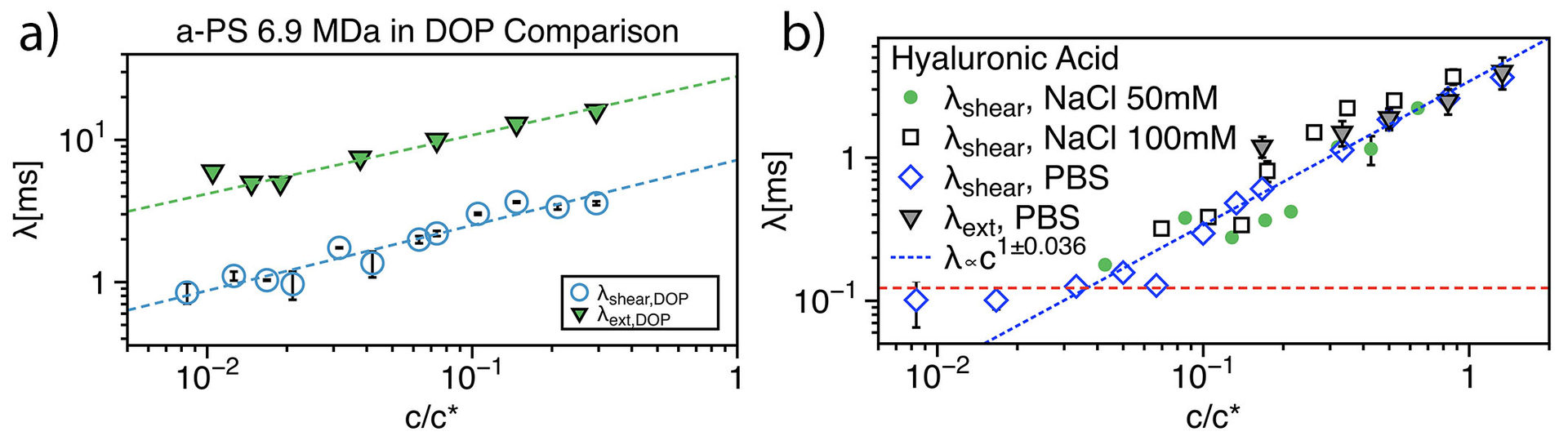
Fig.1 a) Comparison between experimental data derived from x-slot measurements and the μ-rheometer for a-PS in DOP (theta solvent); b) Same as in a) for HA in PBS (good solvent).
3.1.9 Microfluidic-based cell manipulation in viscoelastic fluids
F. Del Giudice, S. Sathish, and A. Q. Shen (Manuscript in Preparation)
Deformability is a critical parameter for discriminating healthy and diseased cells. Several complex microfluidics devices are currently available for separating cells with different deformabilities. Viscoelastic fluids have also been used for particle/cell separation to simplify the microfluidic design. Channel design strongly depends on both fluid rheology and cell deformability. In this work, we study the effect of cell deformability on particle/cell migration perpendicular to the flow, using particles/cells with similar sizes, but different deformabilities, suspended in 2 different viscoelastic fluids (VF1 and VF2) at two different Deborah numbers De, in a straight 150 μm square-shaped microchannel.
At De = 1, both fibroblasts (less deformable) and Jurkat migrate towards the channel centerline (Fig. 1a), in agreement with reported numerical simulations. With smaller diameter, the fraction of Jurkat in the first band should be 50% lower than that of particles. However, deformability-induced force push Jurkat towards the centerline, and the fraction in Fig.1a illustrates a compromise between these two effects. At De = 5, both cells and particles migrate towards the wall (Fig.1e-h). The fraction of deformable fibroblasts and Jurkat on the wall is lower than that for particles due to the effect of the cell deformability. Moreover, higher cell deformability shifts the neutral plane towards the walls, evidenced by some Jurkat being observed in band 2-3.
In conclusion, the effect of deformability on cell migration in viscoelastic fluids at different Deborah number De is studied in a square-shaped microchannel. At low De, cells migrate towards the channel centerline. At high De, cells migrate towards the walls. At low De, cell deformability has a positive effect on cell migration towards the centerline, as clearly observed for the Jurkat. At high De, deformability-induced force and elastic force yield competing effects, as shown for both the fibroblasts and the Jurkat.
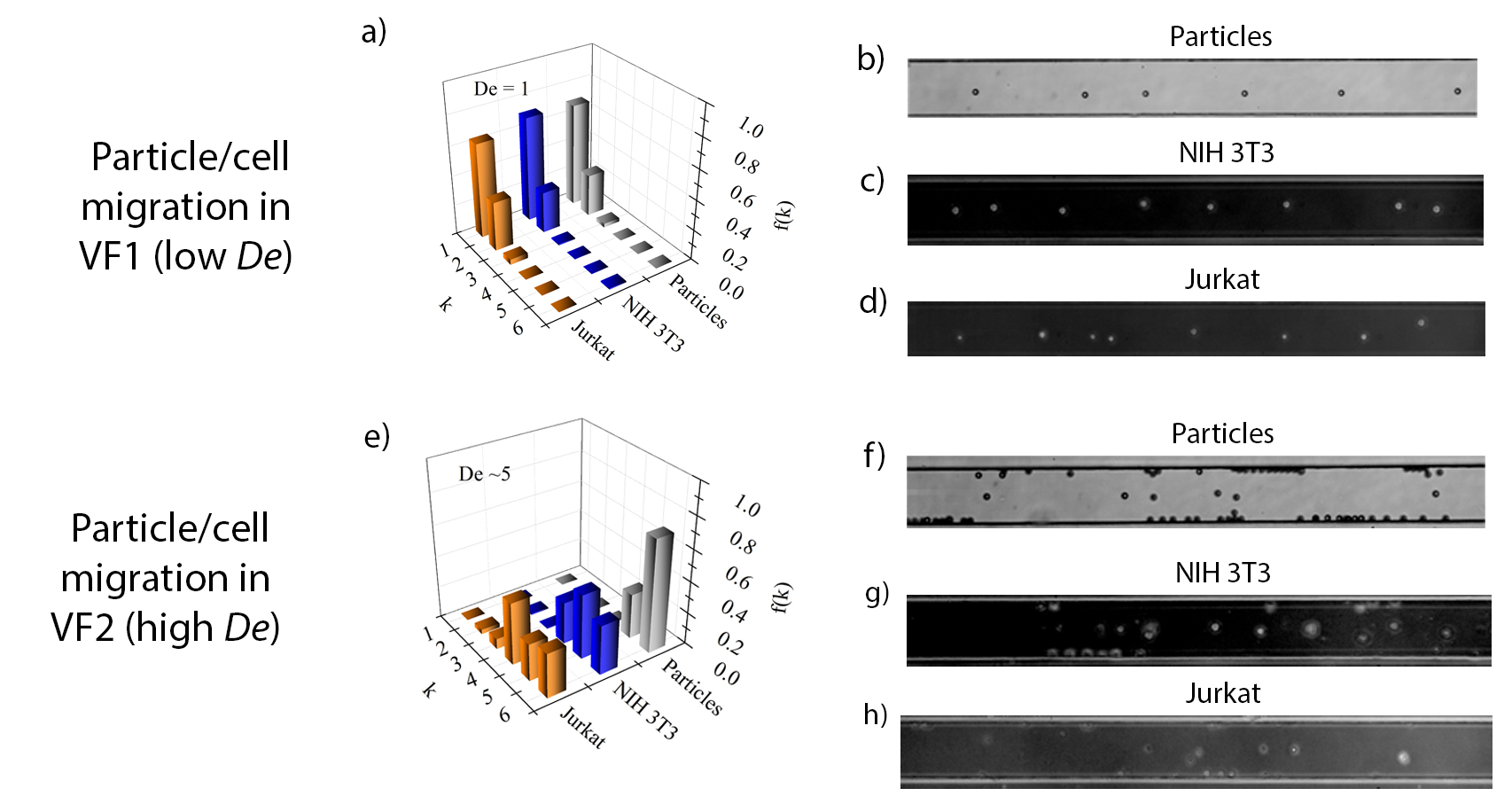
Fig.1: a) Experimental normalized particle distribution f(k), derived from the analysis of the particle velocity [4], for particles and cells in VF1 at De=1. k is the channel band. (b-d) Experimental snapshots at L=5cm from the channel entrance for particles/cells suspended in VF1 at De=1. The analysis of particles/cells velocity indicates that, at De=1, particles and cells migrate to the centerline. e) Experimental normalized particle distribution f(k) as in a), for particles and cells in VF2 at De=5. (f-h) Experimental snapshots as in (b-d) for particles/cells suspended in VF2 at De=5. At De=5, particles and cells migrate towards the walls. Notice that the particles located at the center of the experimental snapshots (f-h) are not in the centerline, because their measured velocities are lower than those predicted around the centerline. Thus, these particles/cells are located mainly on the centerplane in the band k=4-6.
3.1.10 Broadband rheology of rod-like hydroxyethylcellulose water solutions
Francesco Del Giudice, Manlio Tassieri & Amy Q. Shen (Manuscript in Preparation)
Cellulose is the most abundant material on earth. Hydroxyethylcellulose (HEC) is obtained from the chemical reaction of ethylene oxide with wood-based cellulose. It has been shown that HEC behaves as a rod-like polymer when dissolved in water. Thus the opportunity of adopting HEC as model system to address the yet not fully solved dynamics of rod-like polymer solutions. Rod-like polymers are polymers where the single chain in solution assumes a stretched conformation even in static flow conditions. This conformation is achieved when the length of the polymer L is lower (rod-like) or comparable (semi-flexible rod) to the polymer persistence length L_p (a measure of the polymer stiffness). In contrast to the case of fully flexible polymers, for which L>>L_p, the molecular dynamics of rod-like polymer solutions are far from being fully understood and a comprehensive theoretical model is still missing from literature. This is mainly because solutions of semi-flexible polymers have many regimes (spanning several frequency decades) of viscoelastic behavior (requiring many theoretical models), depending on the polymers’ degree of rigidity (i.e. L_p), on their contour length (L), and on the concentration (from dilute to highly entangled regimes). In this work we use five different rheological techniques for the characterization of HEC solutions. In particular, bulk rheology, DLS, DWS, OT and microfluidic techniques have been successfully combined for the study of HEC solutions. Our results show a rarely seen agreement between the viscoelastic behaviors derived through all the different rheological techniques involved. The combination of these techniques allow the study of HEC in almost nine decades of frequency, thus leading to a broadband rheology for the study of the rod-like polymer behavior.
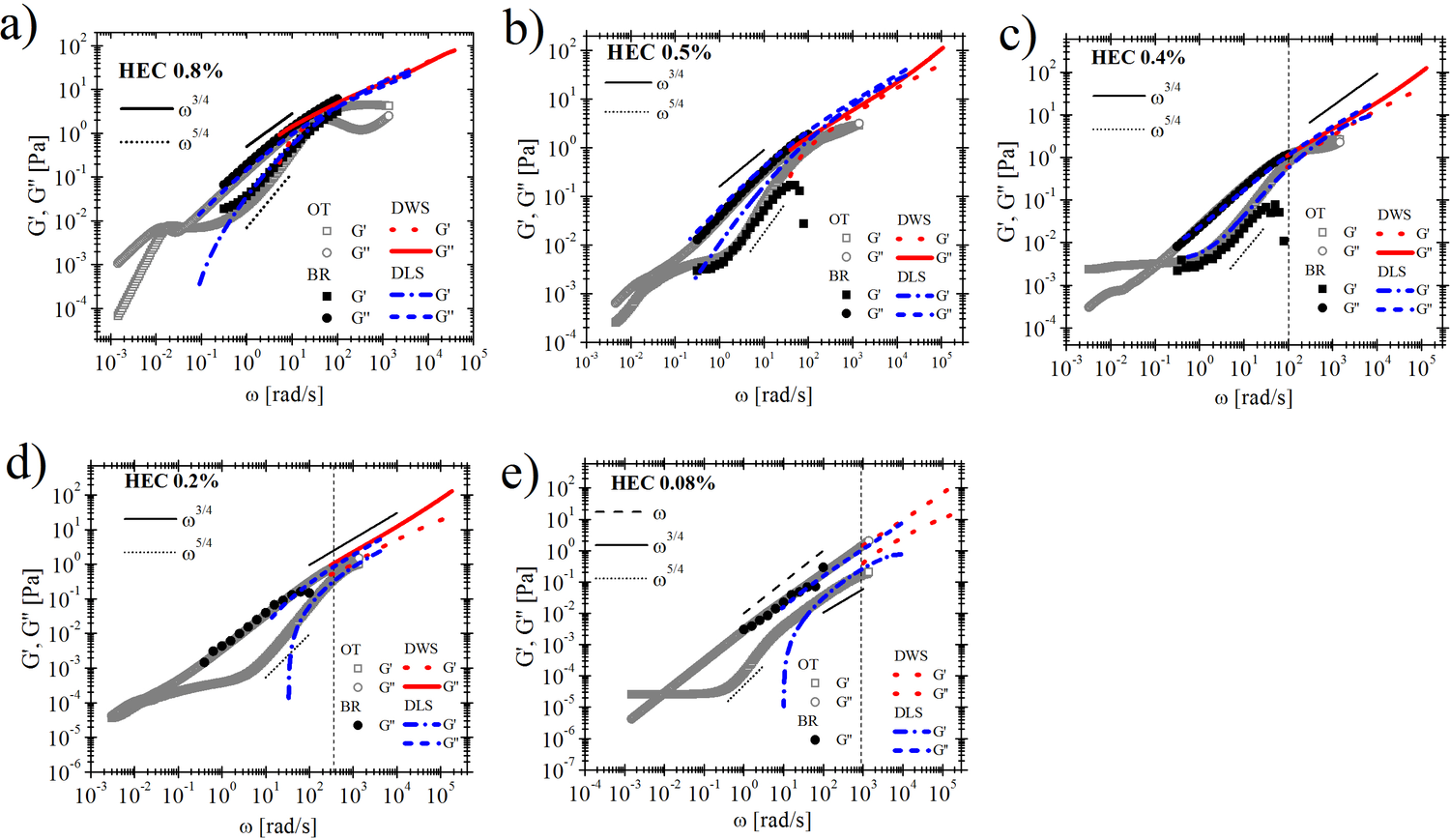
Fig.1: Comparison between the HEC solutions’ linear viscoelastic moduli versus frequency measured with conventional rotational rheometer (filled symbols), with optical tweezers (open symbols), Diffusive wave spectroscopy DWS (red dotted lines), Dinamyc light scattering DLS at 30 degree angle (blue dashed lines) for HEC concentrations: a) 0.8%, b) 0.5%, c) 0.4%, d) 0.2%, e) 0.08%w/w. Notice that the data obtained from both DLS and DWS are vertically shifted of a factor α for all the concentrations.Black solid and dotted lines are the theoretical prediction for rod-like polymers. The black vertical dashed lines in c), d), e) is the inverse of the relaxation time λ measured through the microfluidic technique based on particle migration in square-shaped microchannels.
3.2 Bio-MEMs and BIOSENSING
3.2.1 Micro And Nanopatterned Aminosilanes For Covalent Grafting Of Biomolecules In Multiplexed Microfluidic Bioassays
S. Sathish, S. G. Ricoult, K. Toda-Peters, Aniket Ravan and A. Q. Shen (Manuscript in preparation)
With the growing demand for the miniaturization of biosensors, there is a need for new technologies to achieve efficient surface patterning of biomolecules at the micro- and nanoscale. Since most surface patterning approaches rely on the physisorption of biomolecules, the resulting bond is insufficient to withstand high shear stresses present in micro/nano fluidic devices. Here, we developed a microcontact printing (μCP) approach that enables micro- and nanopatterning of (3-aminopropyl)triethoxysilane (APTES) in a microfluidic device, to covalently graft multiple biomolecules such as proteins and DNA aptamers onto the patterned sites of the device substrate and demonstrated the capability of this integrated platform for immunoassays. The integration of μCP in microfluidic devices opens up new pathways to pattern multiple biomolecules on a single surface to perform multiplexed immunoassay reactions at the micro and nano scales.
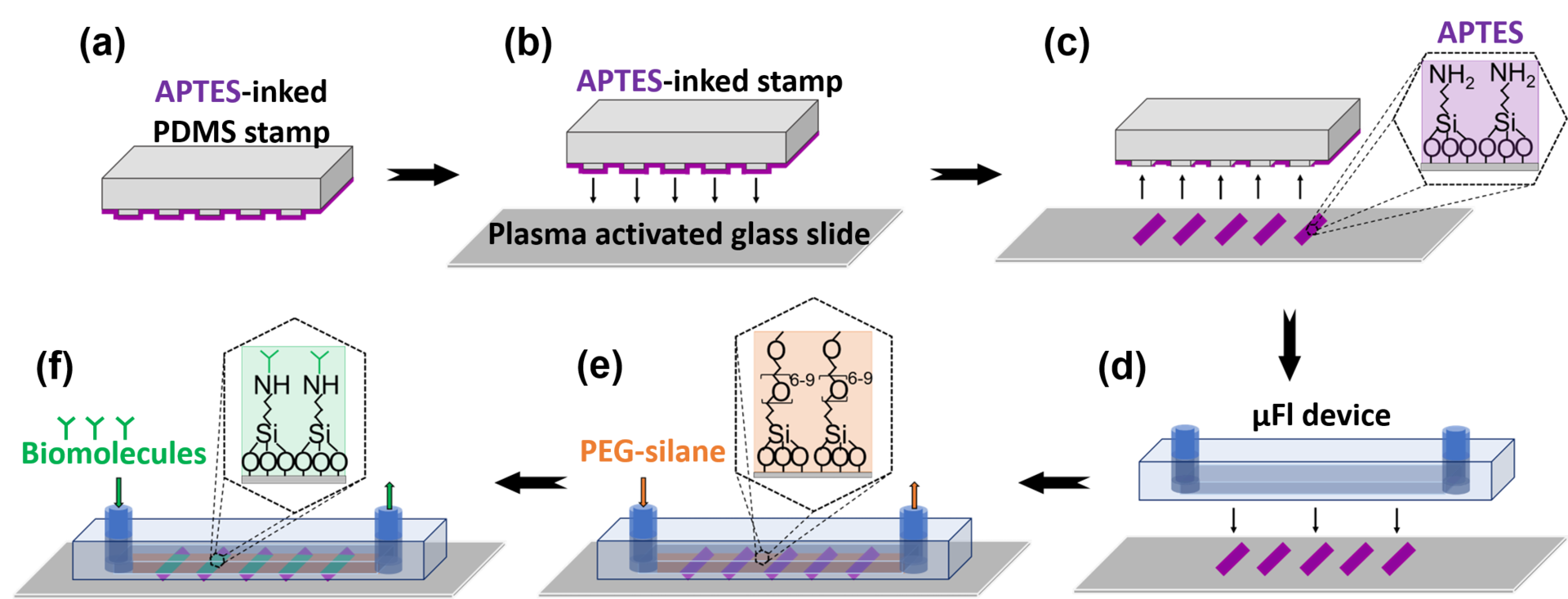
Figure: Patterning chemiadsorbed biomolecules in a μFl device. (a) A PDMS stamp is inked with 1% APTES; (b) The stamp is pressed onto a plasma activated glass slide to (c) transfer APTES. (d) A μFl device is irreversibly bonded onto the patterned glass slide. (e) 1% PEG-silane is flowed through the channels to block non-specific sites. (f) The biomolecules are grafted as (g) micropatterns and (h) nanopatterns onto the APTES pattern via NHS-EDC chemistry. Scale bars are (g) 200 μm and (h) 2.5μm.
3.2.2 Uniform electric field generation in circular multi-well culture plates using polymeric inserts
H.-F. Tsai, J.-Y. Cheng, H.-F. Chang, T. Yamamoto and A.Q. Shen (2016) Scientific Reports 6:26222.
Physiological electric fields play a pivotal role in development, neurogenesis, and wound healing. Applying uniform electric field (EF) in vitro in the physiological range has been achieved in rectangular shaped microchannels. However, in a circular-shape device such as a petri dish, it is difficult to create uniform EF from two electric potentials due to different electrical resistances originated from the length difference between the diameter of the circle and the length of any parallel chord of the bottom circular chamber where cells are cultured. To address this challenge, we developed a three-dimensional 3D computer-aided designed structure in a polymeric insert to create uniform EF in circular shaped multi-well culture plates. A uniform EF with a coefficient of variation (CV) of 1.2% in the 6-well plate can be generated with an effective stimulation area percentage of 69.5%. In particular, NIH/3T3 mouse embryonic fibroblast cells were used to validate the performance of the 3D designed Poly(methyl methacrylate) (PMMA) inserts in a circular-shaped 6-well plate. The CAD based inserts can be easily scaled up (i.e. 100 mm dishes) to further increase effective stimulation area percentages, and also be implemented in commercially available cultureware for a wide variety of EF-related research such as EF-cell interaction and tissue regeneration studies.
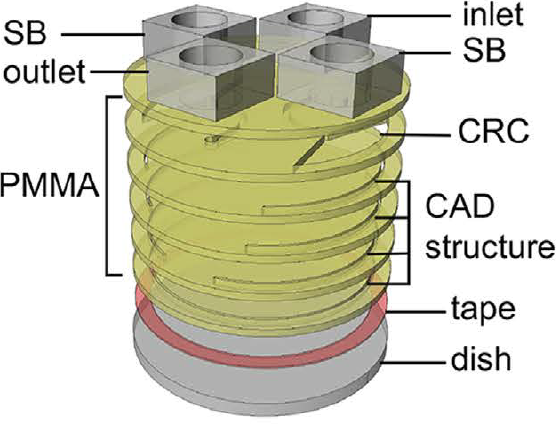
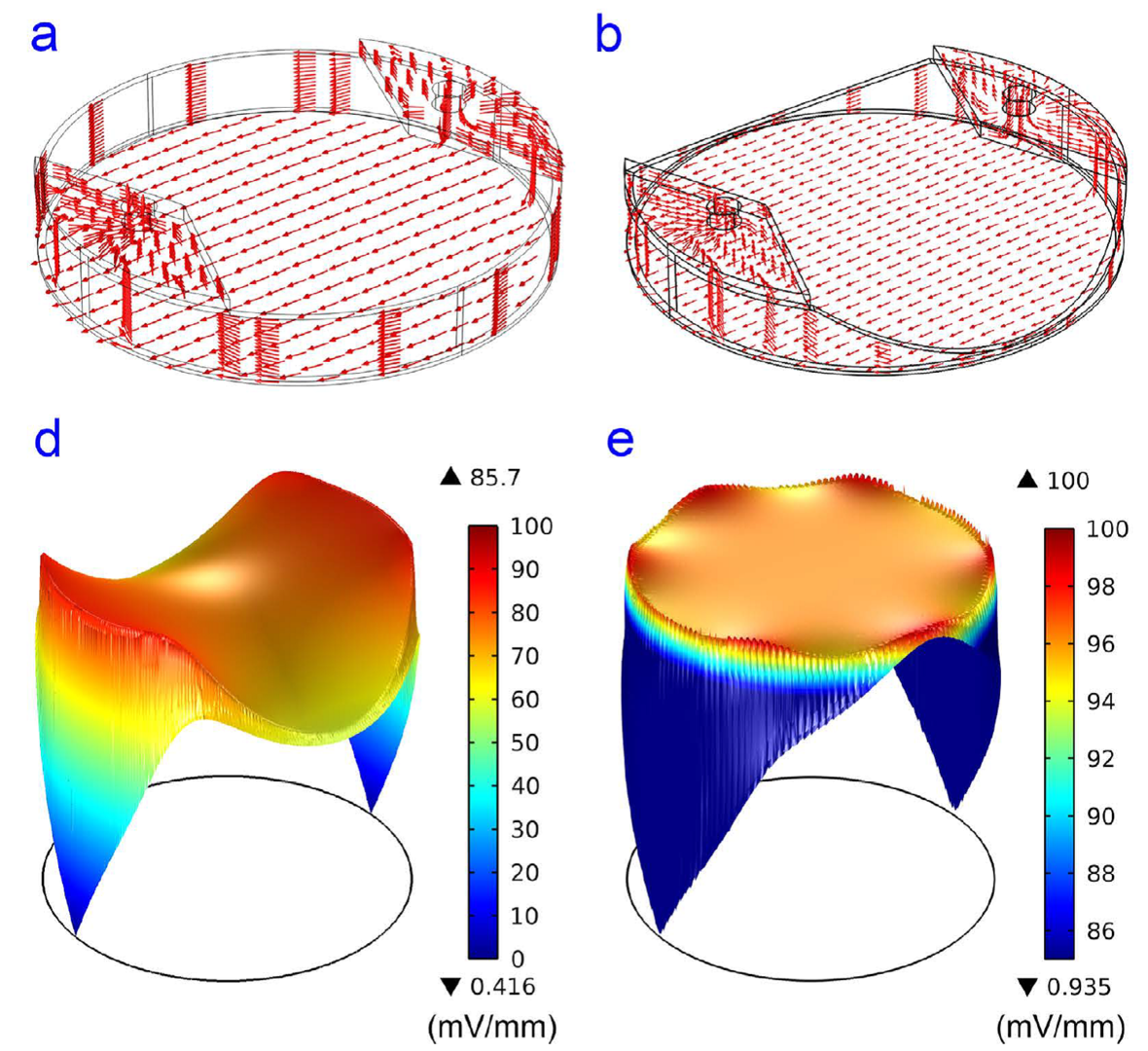
Figure 1 A polymeric insert to create uniform electric field to a circular-shaped area. (A) Schematic of the polymeric insert SB:salt bridge, CRC:current rectifying chambers (B) The numerical simulation results of EF in the inserts (a) the current density in a plain polymeric insert without CAD structures. (b) The current density in a polymeric insert with CAD structure (c) The electric field strength distribution in a plain polymeric insert (d) The electric field strength distribution in a polymeric insert with CAD structure.
3.2.3 An open-electronic/open source programmable temperature controller for molecular biology-on-chip applications
H.-F. Tsai and A.Q. Shen
In molecular biology experiments, temperature control is a great tool to denature and anneal double-stranded nucleic acids. The melting temperature and annealing temperature of a given double-stranded nucleic acids, DNA or RNA, depend on the free energy from the hydrophobic stacking of the base pairs. A precise, programmable, and rapid temperature control is a necessary tool for molecular biology experiments on a microfluidic chip. While expensive commercial products exist, they are in very specific configuration and often use metal or ceramic heat blocks for stable heating. Such heat blocks although are very stable, rapid transition between high and low temperature is difficult. To meet the challenge, we developed a programmable temperature controller that was based on off-the-shelf Arduino electronics and a user-friendly graphical user interface programmed in open-source Python programming language. Taken from the advantage of off-the-shelf electronics, different temperature sensing and heating elements can be chosen by users. Various temperature sensing elements such as thermistor, thermocouples, or diodes can be used with a resolution of 0.001°C. By using a transparent heater (Indium tin oxide coated glass), real-time observation and temperature control can also be enabled by using the temperature controller with microscopes. The stepwise changes in temperature in an experiment can be programmed by users and controlled by proportional-integral-derivative controller in the software. The platform can also be useful for maintaining temperature for cell culture on chip and precise temperature conditioning of photoresists in microfabrication.
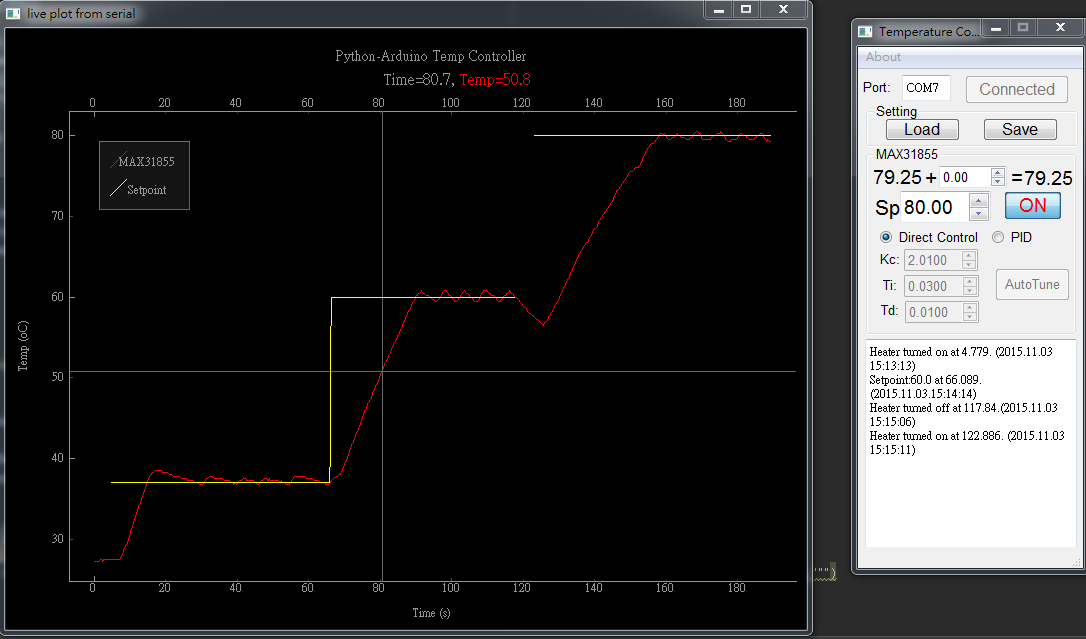
Figure 2 The screenshot of an open source temperature controller for molecular biology.
3.2.4 Novel refractive index biosensing of microcontact printed molecules on lithium niobate
Nikhil Bhalla, Shivani Sathish and Amy Q. Shen (IEEE EMBC 2016 Proceedings)
The development of highly sensitive, selective and multiplex sensors has become an important challenge for diagnosis, prognosis and treatment of diseases. The continuous discovery of the new materials and their properties has provided the basis for many research areas, ranging from materials science to sensing applications. Lithium niobate (LiNbO3) is one of the most multifaceted synthetic materials. It is uniaxial, highly birefringent and has a remarkable combination of piezoelectric, optical properties and photorefractive response. These properties of LiNbO3 have been used to develop a number of surface acoustic wave and electro-optical devices for sensing applications. The optical properties of LiNbO3 can also be exploited to develop a biosensor that detects changes in refractive index upon binding of an analyte. In the past few decades, mostly gold based plasmonic sensors have been used to sense local refractive index changes. Although, gold is a biocompatible material and provides a suitable environment for the attachment of biomolecules, it relies heavily on standard thiol chemistry to bind molecules on its surface. One major disadvantage of thiol chemistry is that it is prone to non-specific attachment of molecules and it is often necessary to create a complex anti-fouling surface.
In this respect, materials compatible with silanes, for instance LiNbO3 provides an advantage of easily creating self-assembled monolayers (SAM) of biomolecules with high specificity. In addition, the photosensitive absorption properties of LiNbO3 are subjected to less losses as compared to plasmons. This makes LiNbO3 one of the potential candidates to replace surface plasmon sensors in the course of evolution of refractive index sensors. We demonstrated the use of lithium niobate as a biosensor that detects local refractive index changes triggered by the presence of biomolecules on its surface. The sensitivity of the sensor was found to be 242±16 nm/RIU. As a case study, we immobilized proteins (IgG antibodies) using micro-contact printing to reveal sensing capabilities of the device. The validated proof of concept will form a foundation for developing lithium niobate based novel optical biosensors.
Figure 1: Schematic of the intrumentation setup for the measurements. The setup consists of a homemade system comprised of a reflection probe (R400-7UV-VIS), halogen light source (HL-2000) and a spectroscope (USB4000-UV-VIS-ES), which were all purchased from Ocean Optics.
3.2.5 Generation of Gradients in Enclosed Geometries
Casey J. Galvin, Makoto Schreiber, Amy Q. Shen
 Microfluidic devices have found widespread use in academic research and are the basis of a number of technologies and products in biotechnology, chemical synthesis and security and defense, among others. The size of scale of microfluidic devices leads to an important role of surface chemistry, and a number of modification strategies have been developed to tailor surface chemistry inside of a microfluidic device. Since the fluidic channels in a microfluidic device are typically enclosed, generating gradients in surface chemistry is difficult. Solving this problem would enable the application of surface chemistry gradients to enhance and control liquid flow in a device, create testing platforms that can detect multiple chemicals, and determine the optimal surface coating for a homogeneously modified device.
Microfluidic devices have found widespread use in academic research and are the basis of a number of technologies and products in biotechnology, chemical synthesis and security and defense, among others. The size of scale of microfluidic devices leads to an important role of surface chemistry, and a number of modification strategies have been developed to tailor surface chemistry inside of a microfluidic device. Since the fluidic channels in a microfluidic device are typically enclosed, generating gradients in surface chemistry is difficult. Solving this problem would enable the application of surface chemistry gradients to enhance and control liquid flow in a device, create testing platforms that can detect multiple chemicals, and determine the optimal surface coating for a homogeneously modified device.
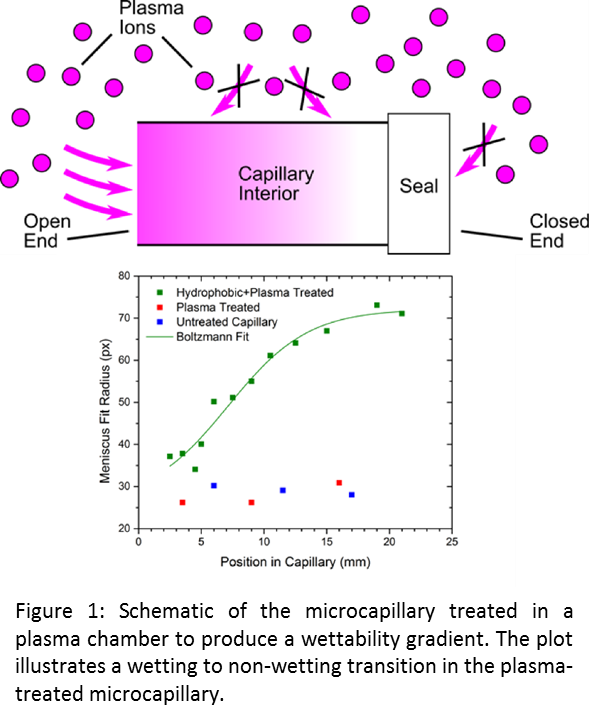
We have demonstrated the ability to produce surface chemistry gradients in enclosed geometries using glass microcapillaries with the interior surfaces modified to be “water hating” (i.e., non-wetting) as a model platform. The gradient is generated by sealing one end of the capillary and treating the entire capillary in a gas plasma chamber, as illustrated in Figure 1. The gas plasma contains reactive ions that diffuse through the open end capillary to the interior surface of the capillary. These plasma ions change the chemistry of the interior surface of the capillary that results in a change in the angle made by a water meniscus in the capillary (i.e., wettability). Due to the asymmetric sealing and high aspect ratio geometry of the microcapillary, a gradient in flux of the reactive ions to the interior surface results in wettability. This gradient was measured experimentally by draining a treated capillary filled with water and filming the draining with a high speed camera. The curvature of the meniscus (a measure of the wettability) was determined by fitting the meniscus to a circle. The radius of this circle is plotted in Figure 1 (green data) as a function of position in the capillary, where a position of zero corresponds to the open end. The data show an increase in the meniscus fit radius (i.e., a decrease in wettability) as one moves further into the capillary, indicating a wettability gradient along the capillary. The sigmoidal shape of the gradient is characteristic of a diffusive gradient, consistent with the proposed mechanism for creating these gradients. The blue and red data are for control samples that were not modified to be non-wetting, and show no apparent gradient, which is expected.
4. Publications
4.1 Journals
- Haward, Simon J., 2015. Microfluidic Extensional Rheometry. BSR Bulletins, 56(3), 65-72
- Haward, Simon J., Poole, Robert J., Alves, Manuel A., Oliveira, Paulo J., Goldenfeld, Nigel, Shen, Amy Q., 2015. Tricritical spiral vortex instability in cross-slot flow. Physical Review E, 93, 031101 (1-5), (doi:10.1103/PhysRevE.93.031101)
- Xu, Lei, Peng, Jinhui, Yan, Mi, Zhang, Di, Shen, Amy Q., 2015. Droplet synthesis of silver nanoparticles by a microfluidic device. Chemical Engineering and Processing: Process Intensification, 102, 186-193, (doi:10.1016/j.cep.2016.01.017)
- Lee, Doojin, Beesabathuni, Shilpa N., Shen, Amy Q., 2015. Shape-tunable wax microparticle synthesis via microfluidics and droplet impact. Biomicrofluidics, 9, 064114, (doi: 10.1063/1.4937897)
- Fang, Cifeng, Lee, Doojin, Stober, Boris, Fuller, Gerald G., Shen, Amy Q., 2015. Integrated microfluidic platform for instantaneous flow and localized temperature control. RSC Advances, 5, 85620-85629, (doi:10.1039/c5ra19944a)
- Chen, Wanyu, Shu, Zhiquan, Gao, Dayong, Shen, Amy Q., 2015. Sensing and sensibility: single-islet-based quality control assay of cryopreserved pancreatic islets with functionalized hydrogel microcapsules, Advanced Healthcare Materials. Advanced Healthcare Materials, 5(2), 223-231, (doi:10.1002/adhm.201500515)
- Cardiel, Joshua J., Furusho, Hirotoshi, Skoglund, Ulf, Shen, Amy Q., 2015. Formation of crystal-like structures and branched networks from nonionic spherical micelles. Scientific Reports, 5, 17941, (doi:10.1038/srep17941)
- Zhao, Ya, Haward, Simon J, Shen, Amy Q., 2015. Rheological characterizations of wormlike micellar solutions containing cationic surfactant and anionic hydrotropic salt. Journal of Rheology, 59, 1229, (doi:10.1122/1.4928454)
- Xu, Lei, Peng, Jinhui, Srinivasakannan, C., Chen, Guo, Shen, Amy Q., 2015. Synthesis of copper nanocolloids using a continuous flow based microreactor. Applied Surface Science, 355, 1-6, (doi:10.1016/j.apsusc.2015.07.070)
- Li, Zhou, Haward, Simon J., 2015. Viscoelastic flow development in planar microchannels. Microfluidics and Nanofluidics, 15, (doi:10.1007/s10404-015-1630-0)
- Galvin, Casey J., Bain, Erich D., Henke, Adam, Genzer, Jan, 2015. Instability of Surface-Grafted Weak Polyacid Brushes on Flat Substrates. Macromolecules, 48, 5677-5687. (doi:10.1021/acs.macromol.5b01289)
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Shen, Amy Q., Microfluidics assisted materials synthesis for biosensing and imaging applications, World Premier International (WPI) Research Center for Materials Nanoarchitectonics (MANA) National Institute for Materials Science (NIMS), Tsukuba, Japan, March (2016)
- Sathish, Shivani, Ricoult, Sebastien G., Shen, Amy Q., Multiplexed Microfluidic Screening Device To Probe Differentiation of iPSCs, CiRA/ISSCR 2016 International symposia. Pluripotency: From Basic Science to Therapeutic applications, Kyoto, Japan, March (2016)
- Shen, Amy Q., Microfluidics assisted materials synthesis for biosensing and imaging applications, Rice University, Houston, USA, February (2016)
- Shen, Amy Q., Novel Microfluidic Platforms for Applications in Biomedicine and Biomaterials Synthesis, The University of California, Berkeley, Berkeley, USA, February (2016)
- Shen, Amy Q., Getting the most from microfluidic platforms in biomedical applications, SPIE Photonics West Bios, San Francisco, USA, February (2016) [Invited lecture]
- Tsai, Hsieh-Fu, Cheng, Ji-Yen, Shen, Amy Q., Uniform electric field generation in circular dishes for cell stimulation by a 3D designed polymeric insert, NanoEngineering for Medicine and Biology (NEMB) Conference 2016, Houston, USA, February (2016)
- Shen, Amy Q., Haward, Simon J., Burshtein, Noa, Toda-Peters, Kazumi, Poole, Robert J., Enhanced microfluidic mixing via a tricritical spiral vortex instability, ASME NanoEngineering for Medicine and Biology Conference, Houston, USA, February (2016)
- Shen, Amy Q., Novel Microfluidic Platforms for Applications in Biomedicine and Biomaterials Synthesis, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, China, January (2016)
- Shen, Amy Q., Microfluidic platforms for biomedical applications, Engineering Department C3-101, Kobe University, Kobe, Japan, October (2015)
- Zhou, Li, Haward, Simon J., Viscoelastic flow development in planar microchannels, 87th Society of Rheology Annual Meeting, Baltimore, USA, October (2015)
- Zhao, Ya, Haward, Simon J., Shen, Amy Q., Rheological characterizations of wormlike micellar solutions containing cationic surfactant and anionic hydrotropic salt, 87th Society of Rheology Annual Meeting, Baltimore, USA, October (2015)
- Ricoult, S. G., Lee, D. J., Ravan, A. S., Shen, Amy Q., CELL DISCRIMINATION AMONG SIMULTANEOUS PRESENTATION OF SUBSTRATE-BOUND AND SOLUBLE PROTEIN GRADIENTS, MicroTAS 2015, Gyongju, South Korea, October (2015)
- Li, Zhou, Haward, Simon J., Viscoelastic flow development in planar microchannels, British Society of Rheology Midwinter Meeting, Glasgow, Scotland, December (2015)
- Walls, Dan, Haward, Simon J., Shen, Amy Q., Fuller, Gerald G., The spreading of sessile drops in miscible environments, American Institute of Chemical Engineers Annual Meeting, Salt Lake City, USA, November (2015)
- Shen, Amy Q., Microfluidic platforms for biomedical applications, OIST Mini Symposium "Current Trends and Emerging Technologies in Nanofluidics and Nanofabrications", Okinawa, Japan, October (2015)
- Ricoult, S. G., Shen, Amy Q., Development and Application of New Micro and nanotechnologies to Study Cell Navigation, OIST Mini Symposium "Current Trends and Emerging Technologies in Nanofluidics and Nanofabrications", Okinawa, Japan, October (2015)
- Lee, Doojin, Beesabathuni, Shilpa, Shen, Amy, Shape controllable wax microparticle generation using microfluidics and droplet impact, The Society of Rheology 87th Annual Meeting, Baltimore, USA, October (2015)
- Haward, Simon J., McKinley, Gareth H., Shen, Amy Q., Testing an elastic instability criterion in a planar elongational flow, 87th Society of Rheology Annual Meeting, Baltimore, USA, November (2015)
- Haward, Simon J., Burshtein, Noa, Poole, Robert J., Shen, Amy Q., Enhanced microfluidic mixing via a tricritical spiral vortex instability, British Society of Rheology Midwinter Meeting, Glasgow, Scotland, December (2015)
- Galvin, Casey, Effect of Nanoscale Confinement on the Stability of Covalently‐grafted, Polymeric Surface Coatings, OIST Mini Symposium "Current Trends and Emerging Technologies in Nanofluidics and Nanofabrications", Okinawa, Japan, November (2015)
- Burshtein, Noa, Haward, Simon J., Poole, Robert J., Toda-Peters, Kazumi, Shen, Amy Q., Enhanced microfluidic mixing via a tricritical spiral vortex instability, 87th Society of Rheology Annual Meeting, Baltimore, USA, November (2015)
- Shen, Amy Q., Microfluidics and Nanofluidics for Drug Carrier Development, IEEE-NANOMED 2015, Hawaii, USA, November (2015) [Invited lecture]
- Shen, Amy, Novel microfluidic platforms for applications in biomedicine and novel materials synthesis, lectrical Engineering and Bioengineering department, University of Hawaii, Hawaii, USA, June (2015)
- Shen, Amy, Microfluidic-assisted hydrogel particle synthesis for encapsulation, imaging, and drug delivery applications, OIST, Okinawa, Japan, May (2015)
- Ripken, Christina, Grossman, Mary, Shen, Amy, Mitarai, Satoshi. Probing marine microorganisms behavior with microfluidic platforms, OIST Mini Symposium "Small Meets Large: Connecting Microfluidics with Marine Ecology", Okinawa, Japan, May (2015).
- Shen, Amy, Getting the most from microfluidic platforms in biomedical applications, Chemical Engineering department, Stanford University, Stanford, USA, April (2015)
- Shen, Amy, Getting the mOIST from microfluidic platforms in applications for biomedicine and novel materials synthesis, Mechanical Engineering Department, University of Minnesota, Minneapolis, Minnesota, USA, April (2015)
- Shen, Amy, Microfluidics assisted bio- and nano-materials synthesis, ASME 2015 4th Global Conference on Nanoengineering for Medicine and Biology, Minneapolis, Minnesota, USA, April (2015)
5. Intellectual Property Rights and Other Specific Achievements
5.1. Grants
1. Amy Shen (PI); 2015--2017; Sysmex Japan, Design and Fabrication of a micro-nano-fluidic device optimized for immunoassays.
6. Meetings and Events
6.1 Seminars
1. Dr. Hervé Elettro (D'Alembert Institute (UMR 7190) CNRS & Université Pierre et Marie Curie)
- Date: August 17, 2015
- Venue: OIST Campus, C016
- Seminar: Elasto-capillary windlass: from spider webs to synthetic actuators
2. Prof. Ben (Tsutomu) Takahashi (Nagaoka University of Technology)
- Date: November 24, 2015
- Venue: OIST Campus, C016
- Seminar: Soft matter handling technology “SWITL”
3. Prof. Anne-Laure Biance (The Institute of Light and Matter)
- Date: December 4, 2015
- Venue: OIST Campus, C015
- Seminar: Rise and decline of soap films
4. Prof. Seung-Wuk Lee (University of California, Berkeley)
- Date: January 14, 2016
- Venue: OIST Campus, C2019
- Seminar: Manufacturing Goes Viral: Biomimetic Self-templating Assembly and Applications
5. Prof. Peter Fischer (ETH Zurich)
- Date: January 15, 2016
- Venue: OIST Campus, C209
- Seminar: Unraveling the defense mechanism of hagfish slime: A rheological approach
- Date: January 21, 2016
- Venue: OIST Campus, D014
- Seminar: Interfacial rheology and reflectivity measurements: Protein adsorption layer, capsule formation, foam and emulsion stability, and biofilms
6. Prof. Aaron Ohta (University of Hawai’i)
- Date: January 26, 2016
- Venue: OIST Campus, C016
- Seminar: Indirect Optical Manipulation of Living Cells
7. Prof. Boris Stoeber (The University of British Columbia)
- Date: February 4, 2016
- Venue: OIST Campus, C015
- Seminar: Microneedles: Tiny Biomedical Tools Targeting the Skin
8. Prof. Norbert Willenbacher (Karlsruhe Institute of Technology)
- Date: March 2, 2016
- Venue: OIST Campus, D014
- Seminar: Capillary Suspensions – a Generic Paste Formulation Concept for Innovative Highly Porous Membranes and Printed Electronics
19. Prof. Howard Stone (Princeton University)
- Date: March 14, 2016
- Venue: OIST Campus, C209
- Seminar: Some Insights into the Mechanical World of Bacteria and Biofilms
- Date: March 15, 2016
- Venue: OIST Campus, C209
- Seminar: New Observations of Particle Motions in Simple Geometries: Examples From Both Low and High Reynolds Numbers
10. Prof. Aaron Wheeler (The University of Toronto)
- Date: March 22, 2016
- Venue: OIST Campus, C016
- Seminar: Introduction to Digital Microfluidics
- Date: March 23, 2016
- Venue: OIST Campus, D015
- Seminar: Digital Microfluidics for Three-Dimensional Cell Culture and Single-Cell Signaling
6.2 Events
1. OIST Mini Symposium "Small Meets Large: Connecting Microfluidics with Marine Ecology"
2. OIST Mini Symposium "Current Trends and Emerging Technologies in Nanofluidics and Nanofabrications"
7. Other
Nothing to report.