Invited Speakers

Prof. Hellmut Haberland

Institute of Physics and Materials Research Institute, University of Freiburg, Germany
The Magnetron Cluster Source, its History and some Applications
Neutral atoms are mainly emitted by the standard magnetron sputtering source, operating at an argon pressure of about 0.01 Pa. For efficient cluster formation, the pressure has to be increased by roughly a factor of hundred. The magnetron cluster source was developed in Freiburg, and is used there today in two different fields: 1) spectroscopy of (mostly) mass selected clusters, and (2) thin film formation by the Energetic Cluster Impact (ECI) technique. The resulting films are strongly adhering and very smooth. Molecular dynamics simulations revealed the underlying physical mechanisms. Unusual combinations of materials can be made: e.g. a tungsten film on Teflon. Several examples will be discussed. The newest version of the source uses an aerodynamic lens to further increase the intensity, and will be used for inner shell spectroscopy of clusters at a Free Electron Laser facility.

Institute of Physics, University of Greifswald, Germany
Formation of nano-size particles and deposition of thin porous films
Nanoparticles (or nano-size clusters) have unique properties which make them suitable for a number of applications. As nano-size clusters we consider aggregates of metal atoms with sizes of up to about 100 nm consisting of typically 104-106 atoms. Nano-size clusters are frequently produced in a gas aggregation-type source. Processes responsible for the growth and transport of metal nano-particles produced in a gas aggregation source based on magnetron sputtering for production of free atoms is discussed. Among the growth processes are nucleation in the early stage, atom absorption, and coagulation in the later stage. The produced nano-size clusters are embedded in a gas flow and leave the aggregation chamber through an orifice where they are accelerated by the outflowing gas. The most probable cluster velocities are in the range of 80-180m/s; measured velocities decrease with cluster size and increase with buffer gas flow. Experimental results are compared with a theoretical model and noted discrepancies are explained by the interaction of clusters with buffer gas atoms outside the aggregation chamber during the passage from the orifice towards the detector. The outflowing nano-size clusters are deposited on Si substrates and some of their properties have been investigated.

Nanoscale Physics Research Laboratory, School of Physics and Astronomy, University of Birmingham, Birmingham B15 2TT, UK
Fulfilling Feynman's vision: arranging the atoms in size-selected clusters and a route to manufacturing
In his famous 1959 lecture "There's plenty of room at the bottom" Nobel Laureate Richard Feynman expressed a vision of making materials by arranging the atoms. Atomic clusters were one of the systems in his thoughts. In this talk I will explore how far we have come in realising the route to new functional materials based on deposition of size-selected atomic clusters with 3D structural control [1,2].
The talk will include a discussion of new efforts to scale-up dramatically the rate of cluster generation, which promise significant future impact. We can now report a cluster beam current of 10 microamps, 4-5 orders of magnitude above a conventional cluster source, with our “Matrix Assembly Cluster Source” (MACS). Various applications in catalysis, metrology and biomedicine will also be addressed.
On the fundamental side one current frontier is the question of the metastability of the clusters themselves. New techniques like aberration-corrected scanning transmission electron microscopy (ac-STEM) are only now being applied to soft-landed, size-selected clusters [2-8]. Dynamical manipulation experiments [6,8], which probe the transformation of metastable isomers into more stable configurations, and reaction-exposure experiments, which probe the response of the nanocluster structures to real catalytic conditions [9], will be treated. Such experiments also provide a body of data to stimulate and constrain computational models and are readily extendable to other sizes and cluster materials including binary systems [10]. The image shows one frame from a dynamical STEM video of an Au923±23 cluster.
1. R.E. Palmer, S. Pratontep and H.-G. Boyen, Nature Materials 2 443 (2003).
2. R.E. Palmer and C. Leung, Trends in Biotechnology 25 48 (2007).
3. Z.W. Wang and R.E. Palmer, Nano Lett. 12 91 (2012).
4. Z.W. Wang and R.E. Palmer, Nanoscale 4 4947 (2012) [Cover].
5. Z.W. Wang and R.E. Palmer, Nano Lett. 12 5510 (2012).
6. Z.W. Wang and R.E. Palmer, Phys. Rev. Lett. 108 245502 (2012).
7. S.R. Plant, L. Cao, F. Yin, Z.W. Wang and R.E. Palmer, Nanoscale 6 1258 (2014) [Cover]; S.R. Plant, L. Cao and R.E. Palmer, JACS 136 7559 (2014).
8. D.M. Wells, G. Rossi, R. Ferrando and R.E. Palmer, Nanoscale 7 6408 (2015) [Cover].
9. K.J. Hu, S.R. Plant, P.R. Ellis, C.M. Brown, P.T. Bishop and R.E. Palmer, JACS (2016): doi 10.1021/jacs.5b08720.
10. C.E. Blackmore, N.V. Rees and R.E. Palmer, Phys. Chem. Chem. Phys. 17 28005 (2015).

Surface Physics & Materials Science Division, Saha Institute of Nuclear Physics, 1/AF Bidhan Nagar, Kolkata-700064, India
Morphologies of soft landed size-selected Cu nanoclusters over different substrates
One of the important aspects of size-selected clusters is the interaction of an individual cluster with other clusters and with the substrate on which these self-assembled clusters reside to form an ultra thin film for possible exhibition of extraordinary properties for applications. In this report, performance of a nanocluster deposition unit with in-situ XPS facility is described. Utilising this facility, the morphologies of size-selected copper clusters deposited on Si, Ge, GaAs and Highly Ordered Pyrolytic Graphite (HOPG) at room temperature and at an elevated temperature of 450oC are studied using AFM, SEM and TEM. Apart from these substrates, clusters are also deposited on amorphous carbon film to study the morphology by TEM. These size-selected clusters are obtained from a gas aggregation type nanocluster deposition unit [1] based on magnetron sputtering ion source. The mass or size of the clusters are selected by using a quadrupole mass filter and in this report, clusters of 3 nm diameter or about 1100 Cu-atoms per cluster is used for deposition in various substrates. The composition of the deposited films were analysed by in-situ XPS attached in the deposition chamber. In order to see the thermal stability of the clusters, the films on different substrates are annealed at 450oC. One of the motivations behind this study is to understand role of diffusion on the morphological evolution over different substrates. Initially low dimensional clusters/adatoms in non-equilibrium states are movable on the substrates and depending on substrate properties these aggregates attain a stable structure. These will be discussed for the films composed of size-selected Cu-clusters at different experimental conditions and their behaviours.
[1] S. Mondal and S.R. Bhattacharyya, Rev. Sci. Instrum, 85, 065109 (2014)
School of advanced engineering, Department of environmental chemistry and chemical engineering, Kogakuin University, Japan
Modeling of Materials for Batteries from Atomistic Level
In this presentation modeling of several kind batteries, e.g. Li-air battery, Li ion battery and fuel cells, were carried out to estimate theoretically battery performance based on the atomistic information. Concerning Li-air battery, design of cathode structure is crucial to determine the battery performance. To model the cathode structure of porous carbon, we investigated a deposition mechanism of discharged product of Li2O2 by first principles molecular dynamics. Our simulation results indicate that deposited Li2O2 cluster diffuse easily on a carbon surface and easily to form a larger cluster. Moreover it is observed the cluster stoop its diffusion by trapping at surface discontinuity. Based on the information regarding molecular dynamics we constructed a numerical model to represent the polarization curve of Li-air battery, which successfully describes the experimental observations. We also applied meso-scale modeling techniques to cathode material to study the relationship to the porous structure and battery performance. Similar multi-scale modeling techniques are applied to polymer electrolyte fuel cells and Li ion battery of which the detailed results will be present at the presentation.

Physics Dept, & Chemistry and Industrial Chemistry Dept., University of Genoa, Italy
Structures and phase transitions in Au-Co nanoalloys
Au-Co nanoalloys have been experimentally produced by different methods [1-3], which have lead to the formation of either phase-separated or intermixed chemical ordering patterns. The formation of intermixed nanoalloys has fostered some speculation about an increase of equilibrium miscibility at the nanoscale, because the Au-Co system presents a very weak miscibility in bulk crystals. This is still an open issue, which can be clarified by the use of computer simulations.
Here, by means of DFT and atomistic calculations we demonstrate that [4]
– intermixed configurations are indeed metastable, while most stable configurations are either core-shell or quasi-Janus – phase-seperated structures persist well above room temperature, without indication
of appreciable increases of miscibility up to the melting temperature
– a small number of Co impurities in Au nanoparticles can cause the structural transition from decahedral/fcc structures to icosahedra with reconstructed surface and then to perfect Mackay icosahedra
Preliminary results for Au-Fe are also presented.
[1] A. Mayoral et al., Chem. Commun. 51, 8442 (2015)
[2] L.E. Marbella et al., Adv. Funct. Mater. 24, 6532 (2014)
[3] V. Dupuis et al., Phys. Chem. Chem. Phys. 17, 27996 (2015).
[4] E. Panizon, J.-P. Palomares-Baez, R. Ferrando, in preparation

Instituto de Ciencia de Materiales de Madrid, Spain
One-Step Generation of Alloyed, Core@Shell and Core@Shell@Shell Nanoparticles by gas aggregation
The fast growing nanotechnology is demanding high-quality multifunctional nanoparticles for the fabrication of more complex materials [1]. In such context the gas aggregation synthesis of nanoparticles has merged as a promising technique for the fabrication of well-defined nanoparticles not only for fundamental studies, but also for targeted applications [2-4] In this talk we will present a relatively new fabrication route based on the magnetron sputtering gas aggregation source that allows the generation of nanoparticles with controllable and tunable chemical composition and structure. This technique, called Multiple Ion Cluster Source (MICS), is an evolution of the standard Ion Cluster Sources (ICS) [5]. Through examples, we will show that the MICS technique allows the one-step generation of alloyed [6], core-shell and core@shell@shell nanoparticles [7]. In particular, we will show that the atoms of the core and the shell of the nanoparticles can be easily inverted, avoiding the intrinsic constraints of chemical methods. Finally we will address the up-scaling of the technique to the pre-industrial stage, which remains a long lasting and challenging topic.
[1] U. Simon, Nature Materials 12, 694 (2013).
[2] A. Mayoral et al., Chem. Comm. 51, 8442 (2015).
[3] M. Benelmekki et al., Nanoscale 6, 3532 (2014).
[4] E. R. Zubarev, Nature Nanotechnology 8, 396 (2013).
[5] H. Haberland, et al., Z. Phys. D: At., Mol. Clusters 20, 413 (1991).
[6] L. Martínez, et al., Langmuir 28, 11241 (2012).
[7] D. Llamosa, et al., Nanoscale 6, 13483 (2014).

Department of Physics, Aristotle University of Thessaloniki, GR-54124 Thessaloniki, Greece
Computational investigations of nanostructures: from strain fields to atomic scale phenomena.
Semiconductor heterostructures offer the possibility of electronic property engineering and thus find their way in numerous optoelectronic applications. Core/shell nanoparticles(NPs) and nanowires(NWs), in which the core and the shell are of different materials, offer a way to relax the strain induced by the lattice parameter mismatch and create defect-free interfaces, owing to their large surface to volume ratio. Interatomic potential based Molecular Dynamics(MD) simulations as well as ab initio calculations are employed to investigate the structural, thermal and electronic properties of polar GaN/AlN core/shell nanowires. The bandgaps of the nanowires are scrutinized through ab initio simulations of 103 atoms.
The influence of strain on the energetics and the electronic properties of NWs consisted of a GaN base part followed by a superlattice part of successive InxGa1-xN nanodisks(NDs) separated by GaN spacers is investigated by MD simulations as well as by ab initio calculations.
It has been found by both simulation approaches that among three possible types of strain (biaxial, hydrostatic and uniaxial), the biaxially strained NW superlattice is the one with the lowest excess energy for all indium concentrations. It is concluded that hydrostatic and biaxial strain components should be both considered in the embedded NDs and they are of different physical origin.
Prof. Junichi Murakami

Graduate School of Science and Engineering, Saitama University, Japan
Low-temperature formation of NH3 from N2, mediated by small transition-metal clusters
It is known metal clusters have unique catalytic properties that do not exist for bulk surfaces of corresponding metals. In nature, such clusters, containing a few atoms of transition-metal elements, are found at the reaction centers of metalloenzymes. Owing to the clusters, the enzymes can catalyze difficult chemical reactions under mild conditions. This leads us to expect that, if nanoclusters comprising a few transition-metal atoms are prepared and supported on a surface, they may also mediate difficult chemical reactions under mild conditions.
We have explored the possibility of nitrogen fixation (conversion of N2 to NH3) under mild conditions by transition-metal clusters, which is done in nature by the enzyme nitrogenase. We have found N2 is activated at low temperatures by small tungsten clusters deposited on a graphite surface. The activated N2 reacts with H2O and is converted to N2O or NH3. We also found recently that N2 is activated on N-contaminated palladium surfaces at room temperature, which we believe is due to N2 adsorption on cluster-like structures of the surface. Since Pd easily dissociates H2, the activated N2 is readily hydrogenated with H atoms resulting from H2 on the same surface, leading to the formation of NH3. This is the first example of N2 reduction to NH3 with H2, catalyzed by a heterogeneous system at low temperatures.

Nanoparticles by Design Unit, OIST, Japan
A comprehensive theoretical study of metallic nanoparticle coalescence
Metallic nanoparticles offer an attractive alternative to their bulk counterparts for many applications such as catalysis, biotheranostics, gas sensing, etc. Their performance depends strongly on surface structure; therefore, coalescence can play an important role, as it determines this structure. With this in mind, we performed classical molecular dynamics (MD) simulations to study the mechanisms that govern the coalescence of various single or hybrid metallic nanoparticles.
Our MD simulations captured the propagation of a crystallisation wave through amorphous Pd nanoparticles, even at room temperature. In contrast, under the same conditions, Ta nanoparticles remained amorphous. An almost-epitaxial alignment, assisted by the formation of twins and surface protrusions, was observed in crystalline nanoparticles of both species. We elucidate the protrusion creation through a shearing mechanism and by tracking the glide of temporarily emergent interface dislocations.
Based on our simulation data, we propose a rigorous yet simple analytical method which describes element-independent coalescence behaviour, emphasising only on the predominant dependence of neck formation on temperature and size-dependent nanoparticle melting points. Its simplicity makes our model a suitable starting point for the development of a meso-scale simulation technique that can describe the growth of porous films and allow for their bespoke design.
Finally, we utilise this model to demonstrate that controlled simultaneous co-deposition of pure Ag and Cu nanoparticles allows designing Ag-Cu nanoparticles of desired configurations via coalescence mechanisms.

Department of Materials Science and Engineering, Stanford University, Stanford, CA 94305-4045, USA
Kinetics of Hydrogen Storage Reactions in Thin Film and Nanostructured Materials
With the intensifying global need for alternative energy, there is strong interest in new approaches for sustainably storing energy. Hydrogen is attractive for energy storage due to its high energy density, the ability to be produced by electrolysis from intermittent alternative electricity sources, as well as the potential for direct hydrogen production from sunlight. However, storing hydrogen in a low cost, lightweight, and efficient manner remains a challenge. One possible approach is storing hydrogen with reversible reactions to form a metal hydride. This can produce a volumetric hydrogen density greater than that of pure solid hydrogen.
The challenge for hydrogen storage materials is to find lightweight materials that can reversibly react with hydrogen at reasonable pressures, temperatures and rates. Mg has many attractive features as a hydrogen storage material, but the hydride it forms is too thermodynamically stable and it has sluggish kinetics. Here we report on approaches to tune the reaction thermodynamics as well as to study and control the reaction kinetics. Our work in Pd-caped Mg films reveals the importance of an interfacial alloy in controlling reaction thermodynamics. We also show that the reaction kinetics in the blanked-coated films transitions from an interface-limited to a diffusion limited reaction.
To study the hydride formation kinetics in a controlled way, we have designed a unique ‘nanoportal’ structure of Pd nanoparticles deposited on epitaxial Mg thin films, through which the hydride will nucleate only under Pd nanoparticles. We propose a growth mechanism for the hydrogenation reaction in the nanoportal structure, which is supported by scanning electron microscopy (SEM) images of hydrogenated samples exhibiting consistent results. Interestingly, the grain boundaries of Mg films play an important role in hydride nucleation and growth processes. Kinetic modeling based on the Johnson–Mehl–Avrami–Kolmogorov (JMAK) formalism seems to agree with the two-dimensional nucleation and growth mechanism hypothesized and the overall reaction rate is limited by hydrogen flux through the interface between the Pd nanoparticle and the underlying Mg film. The fact that in our structure Mg can be transformed completely into MgH2 with only a small percentage of Pd nanoparticles offers possibilities for future on-board storage applications.
The challenge for hydrogen storage materials is to find lightweight materials that can reversibly react with hydrogen at reasonable pressures, temperatures and rates. Mg has many attractive features as a hydrogen storage material, but the hydride it forms is too thermodynamically stable and it has sluggish kinetics. Here we report on approaches to tune the reaction thermodynamics as well as to study and control the reaction kinetics. Our work in Pd-caped Mg films reveals the importance of an interfacial alloy in controlling reaction thermodynamics. We also show that the reaction kinetics in the blanked-coated films transitions from an interface-limited to a diffusion limited reaction.
Prof. Boris M. Smirnov

Joint Institute for High Temperatures, Izhorskaya 13/19, Moscow 127412, Russia
Generation and application of metal fractal fibers.
The analysis of processes is made for transformation of a metal micron-size particle in a fractal fiber if this particle is located at the beginning in a gas flow such that the particle weight is equalized by the friction force and this particle hangs in the flow in the course of its vaporization. The particle is heated by an external source, as gas discharge, UHF radiation or laser radiation, and the degree of this action is the particle temperature at which it is evaporated and transfer a heat to the flow of a buffer gas (argon). The flow temperature decreases along the flow and the character of particle transformation is demonstrated in Figure 1, and typical times of these processes are given in Figure 2.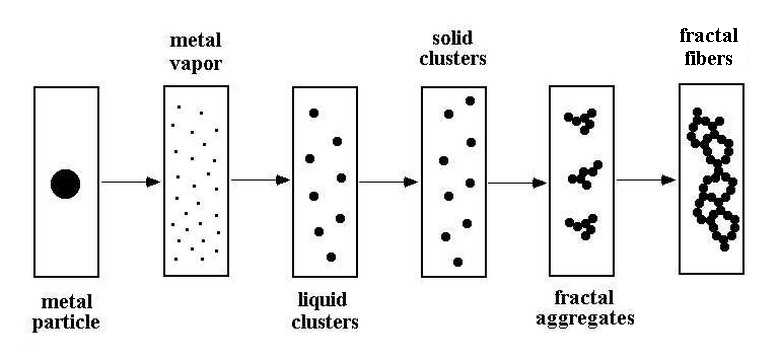
The latter process proceeds in a separate chamber under the action of an external electric field, and separate fractal fibers have consist of solid nanoclusters with centimeter lengths in the field direction and micron sizes in the transverse directions. These metal structures may be used as a catalyzer for chemical processes in gas flows, as well as for air purification and destruction of bacteria.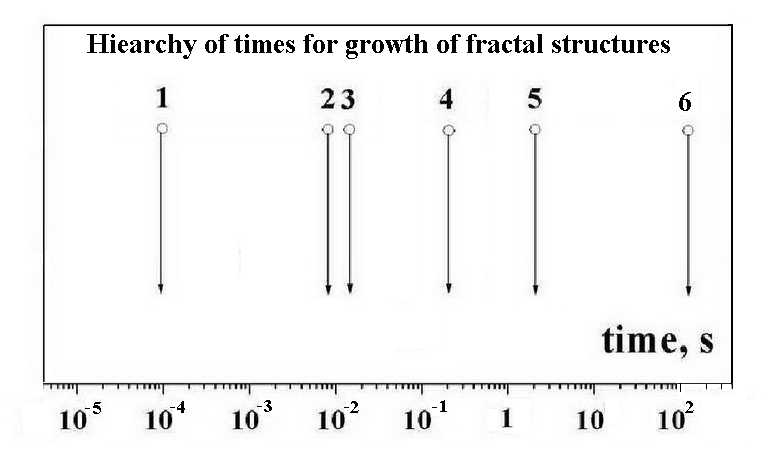
1 – formation of liquid clusters, 2 – cluster transition in the solid state, 3 - transport of atoms to a flow region, 4 – microparticle fall, 5 - microparticle vaporization, 6 - formation of fractal aggregates and fractal fibers.

Helsinki Institute of Physics and Department of Physics, University of Helsinki, Finland
Formation mechanisms of iron nanocubes in magnetron sputtering inert gas condensation
Why do iron nanocubes form during condensation process instead of usual spherical or close-to-spherical shapes? It is counter-intuitive from the surface minimization considerations. We address this question by applying the combination of three different techniques: experiment, Molecular Dynamics and Kinetic Monte Carlo simulations. By these methods we study kinetics of the formation mechanism of iron nanocubes. Our experiments as well as computer simulations indicate that the cubic shape of iron nanoparticles is explained by the difference in the kinetic growth modes of (100) and (110) surfaces, rather than the surface formation energetics. Our results are in good agreement between the different methods, showing that the final shape is defined by condensation temperature in combination with deposition rate. We present also the full deposition rate--temperature diagram of iron nanocluster shapes as well as an analytical model predicting the temperature and deposition rate evolution in nanoparticles. Combined together, the diagram and the model can be used to tune the desired final shape of the grown iron nanoparticles.

Computational Heterogeneous Catalysis, University of Otago, Dunedin, New Zealand
Abstract
Metal nanoclusters are frequently utilized as heterogeneous catalysts due to their high catalytic activity. This high activity is due to the high surface area to volume ratio, large number of undercoordinated atoms and unique electronic properties compared with the bulk material. The catalytic activity is highly dependent on the size and specific shape of the nanocluster. Thus it follows that to have a thorough understanding of the catalytic activity and to design optimal catalysts, detailed understanding is required of the relationship between size and shape of nanoclusters, in addition to knowledge of the activity of various active sites.
In the present work, firstly the relationship between shape and activity of metal nanoclusters will be discussed. Examples will be given for H2 evolution on Pt nanoclusters and N2 dissociation on Ru nanoclusters. Secondly, the influence of size and temperature on the relative stability of icosahedral, FCC and decahedral Au nanoclusters between 600-4000 atoms will be presented and discussed in the context of rational catalyst design.

Faculty of Science, University of the Ryukyus, Japan
Nanostructured M-B-H hydrogen storage materials by milling hydrogenation
Ball milling is useful technique for hydrogenation. The advantages of milling hydrogenation are: (1) accelerating hydrogenation; (2) realizing hydrogenation RT in low hydrogen pressure (because of ΔG = ΔH – TΔS); (3) proceeding local hydrogenation on the edge or surface such as carbon and; (4) accelerating low dehydrogenation temperature due to nanostructure of hydrogenated state. In this study, several hydrogen storage systems using milling hydrogenation of boride have been demonstrated. The mixtures of ScH2 and metal borides (MBn; M = Mg and Ca) were hydrogenated by milling under H2 pressure at RT. ScH2-MgB2 and ScH2-CaB6 mixtures after milling desorbed 3.4 and 2.3 mass% of H2, respectively, with peaks below 300 °C, while milled MBn desorbed 3.1 and 1.7 mass% of H2, respectively. TEM, XAS, and NMR revealed that ScH2-MgB2 and ScH2-CaB6 formed ScB2 and borohydrides (and MgH2 when M = Mg) after milling. In the case of 2CaH2-CaB6 which is dehydrogenated state of Ca(BH4)2, two starting conditions, which are (a) commercial samples and (b) obtained from Ca(BH4)2 dehydrogenation, were used for milling hydrogenation. As a result, sample (b) obtained better yield as ~50% than sample (a) as ~33% due to smaller crystalline size of CaB6 in (b) than that of (a).

Faculty of Physical Sciences, University of Iceland, Iceland
On the structure and catalytic activity of transition metal nanoparticles
''Results of calculations on the atomic structure of Au, Pt and Cu nanoparticles with 100-2000 atoms will be presented. For particles with up to ca. 500 atoms, global optimization has been carried out using the GOUST method which involves finding saddle points on the energy surface. An estimate of the mechanism and rate of structural transitions of the particles is, therefore, also obtained. For larger particles, a systematic procedure for constructing asymmetric as well as symmetric decahedral and FCC structures has been used to identify the lowest energy arrangement of the atoms. Finite temperature effects have been included within the harmonic approximation. The catalytic activity of edges and facets of the nanoparticles has been studied using periodic models and nudged elastic band method for finding minimum energy paths. Results on the hydrogen evolution and CO2 eletrochemical reduction reactions will be presented.''

Zernike Institute of Advanced Materials, University of Groningen, The Netherlands
Core-Shell nanoparticles towards functionality: H- storage, Magnetism, and wetting Phenomena
Nowadays monometallic and bimetallic nanoparticles (NPs) have emerged as key materials for important modern applications in plasmonics, catalysis, biodiagnostics, energy storage, and magnetics. Consequently the control of NP structural motifs with specific shapes provides increasing functionality and selectivity for related applications. However, producing bimetallic NPs with well controlled structural motifs still remains a formidable challenge. In my talk I will address first the issues of a methodology for gas phase synthesis of bimetallic NPs with distinctively different structural motifs ranging at a single particle level from a fully mixed alloy to core–shell, to onion (multi-shell), and finally to a Janus/dumbbell with the same overall particle composition, as well as emphasis will be given to Hydrogen storage systems involving Mg-M (Cu, Ni, Ti). Furthermore, it will be addressed issues for Fe/Fe-oxide core shell nanoparticles and possible structures that can be obtained via gas phase systhesis with prospect for biomedical applications. Finally, I will discuss issues related to roughness controlled superhydrophobicity on single nanometer length scale with metal/non-metal (Cu, C, GeSbTe) nanoparticles Independent of the exact details, our approach to alter the wetting state and induce droplet trapping is straightforward without involving special micro/nano (hierarchical) structuring facilities but instead using direct single distinct nanoparticle deposition onto any type of surface (hydrophilic and/or hydrophobic).
Zernike Institute of Advanced Materials, University of Groningen, The Netherlands
Tailor-made gas phase based nanoparticles with functional properties
Using a home modified Mantis dedicated nanocluster source we have the possibility to produce nanoparticles (NPs) of a great variety of materials with relatively small size dispersion and with properties that can be novel and different from their bulk counterpart. The system works on the principle of inert gas condensation and magnetron sputtering. We have produced a whole range of different NPs with size and motif control. • Covalent bonded NPs, in particular carbon; • Metallic NPs: Cu1, Fe, Mg, Mo, Co, Al, Ag, Nb, Ti, Pd; • Semiconductor NPs, in particular Ge; • Bimetallic NPs: MgNi, MoCu, MgTi with several compositions; • Ternary alloy NPs, e.g. GeSbTe with several compositions and with amorphous and crystallinity control. The particles can be deposited on most surfaces provided they have good vacuum compatibility. The applications range from novel: • Wetting phenomena; Cu NPs covered surfaces giving rose petal effect2; • Bimetallic Mo-Cu NPs which are bulk immiscible but in NPs fully miscible3; • Bimetallic NPs for hydrogen storage4: MgNi, MgTi, MgCu; • Magnetic NPs: Fe-Fe3O4 core-shell particles for medical applications.
1. Brink, G. H. ten, Krishnan, G., Kooi, B. J. & Palasantzas, G. Copper nanoparticle formation in a reducing gas environment. J. Appl. Phys. 116, 104302 (2014).
2. Ten Brink, G. H., Foley, N., Zwaan, D., Kooi, B. J. & Palasantzas, G. Roughness controlled superhydrophobicity on single nanometer length scale with metal nanoparticles. RSC Adv. 5, 28696–28702 (2015).
3. G. Krishnan, M.A. Verheijen, G.H. ten Brink, G. Palasantzas, B.J. Kooi, Tuning structural motifs and alloying of bulk immiscible Mo-Cu bimetallic nanoparticles by gas-phase synthesis, Nanoscale 5, 5375-5383 (2013).
4. Krishnan, G. et al. Synthesis and exceptional thermal stability of Mg-based bimetallic nanoparticles during hydrogenation. Nanoscale 6, 11963-11970 (2014).
Department of Physics, University of Helsinki, Finland
Explicit simulation of nanocluster formation under gas phase condensation
The ground state equilibrium structure of nanoclusters has been studied extensively by several computer simulations methods. However, in experimental nanocluster synthesis, it is not a priori clear whether the clusters will actually reach the thermodynamic equilibrium state. In this talk, I will describe our work on simulating the formation of nanoclusters by explicit modelling of how the atoms join together and cool down in a gas phase carrier medium, similar to the conditions in experimental gas phase condensation cluster sources. These simulations allow following the thermal evolution of the clusters during the formation process, showing how the clusters during the first stages of the nucleation heat up close to the boiling point, before starting to cool down by interactions with the gas atoms [1]. Depending on the cooling rate (related to the gas density), the clusters may not have time to reach the equilibrium state, but remain in some metastable condition. Comparing fcc metals with semiconductors like Si and Ge, our results show that metals crystallize easily and hence can reach a final state close to the ground state one, while semiconductors tend to remain amorphous [2], and sometimes remain in highly non-spherical states formed during cluster agglomeration [3].
[1] E. Kesälä, A. Kuronen, and K. Nordlund, Phys. Rev. B 75, 174121 (2007)
[2] J. Zhao, C. Cassidy, P. Grammatikopoulos, V. Singh, K. Aranishi, M. Sowwan, K. Nordlund, and F. Djurabekova, Phys. Rev. B 91, 035419 (2015)
[3] A. Harjunmaa and K. Nordlund, Comput. Mater. Sci. 47, 456 (2009).
Nanoparticles by Design Unit, OIST, Japan
Gas Phase Design and Synthesis of Nanoparticles for Nanotechnology and Bio-Medical Applications
In this talk I will address the synthesis and growth of nanoparticles using gas-aggregated co-sputtering from elemental source targets, and the integration of these nanoparticles into innovative technologies.
Co-sputtering is a promising method of producing uniform hybrid nanoparticles with tailored composition and microstructure. I will demonstrate control over size, chemical composition, shape and other parameters to tune the physical and chemical properties of nanoparticles for particular applications.
Poster Session Participants
- Dr. Kengo Aranishi
Ni-Pt NPs Deposited on CuO Nanowires & Catalyzed Hydrolysis Reaction of Ammonia Borane
- Ms. Ekaterina Baibuz
Kinetic Monte Carlo simulations of surface diffusion during condensation processes of BCC metal nanoparticles.
- Mr. Daniele Bonventre
Vibrational Properties of Nano-alloys
- Mr. Gert ten Brink
Roughness controlled superhydrophobicity on single nanometer length scale with metal nanoparticles.
- Mr. Bin Chen
Size-dependent crystallization of Ge2Sb2Te5 phase-change nanoparticles
- Dr. Soumen Giri
Nickel-based cathode catalyst for efficient bio-electricity generation
- Ms. Brenna Marie Gibbons
Bimetallic Silver-Copper Nanoparticles for the Oxygen Reduction Reaction
- Dr. Elvar Jonsson
Self-interaction corrected DFT studies of magnetic nanoclusters
- Mr. Ioannis Karakostas
Molecular dynamics investigation of GaN nanocrystals formation in SiO2 and their influence on thermal conductivity.
- Dr. Jaroslav Kousal
Gas aggregation sources as effective tools for production of nanoparticles.
- Mr. Jiri Kratochvil
Application of Ag nanoparticles prepared by GaS for antibacterial nanocomposite coatings.
- Dr. Sushant Kumar
Hydrogenation of Mg nanofilms using size-selected Pd nanoparticles
- Mr. Peng Mao
Synthesis of cluster-assembled nanostructured materials applied for light extraction of light-emitting devices.
- Dr. Lidia Martínez
Scaling of Gas Aggregation Sources
- Dr. Jean-Gabriel Mattei
TEM investigations of trimetallic nanoparticles
- Mr. Shyamal Mondal
Oxidation behavior of supported Cu nanoclusters
- Mr. Emanuele Panizon
Strain-induced restructuring of the surface in core@shell nanoalloys
- Mr. Theodoros Pavloudis
Hydrogen incorporation in Pd/Mg nanostructures and interfaces
- Mr. Shuhei Saito
Effect of Surface Composition of Pt2Ru3 Nanoparticle on CO adsorption Investigated by the Material Informatics Integrated Computational Chemistry
- Mr. Sho Sashida
Chemical treatment of nanostructured B-H materials towards synthesis of ammonia borane
- Dr. Stephan Steinhauer
In situ transmission electron microscopy of Fe nanocubes
- Dr. Vidyadhar Singh
Heterogeneous gas-phase synthesis of metal oxide-encapsulated noble metal nanocatalysts
- Mr. Masahiro Soeno
Molecular Modeling of Electrochemical Reaction of Lithium Oxides during Charging Li-O2 Battery
- Ms. Evropi Toulkeridou
Extended Cluster Heating Model for Nanoparticle Coalescence
- Dr. Jerome Vernieres
Iron Nanocube Yield Maximisation via Size Selection sans Post-Growth Mass Filtration
- Ms. Melissa Rachel Wette
Structural Characterization of AgCu Nanoalloys
- Ms. Dawn Wells
The Atomic Structure and Metastability of Gold and Silver Nanoclusters
- Ms. Lijuan Xing
Synthesis and Morphology of Iron-Iron Oxide Core-Shell Nanoparticles Produced by High Pressure Gas Condensation
- Mr. Junlei Zhao
Surface Segregation in Chromium-Doped NiCr Alloy Nanoparticles – Metropolis Monte Carlo Approach.



