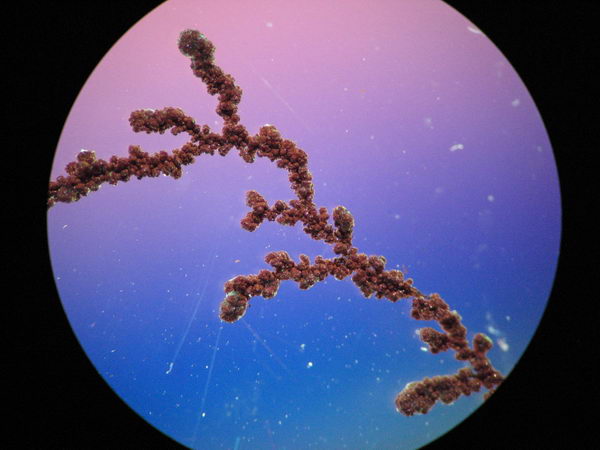
DLA, Electroplating and oscillating reaction lab
Finally, we had our last laboratory experiment in “Inorganic Electrochemistry” class, during which we collected some entertaining experiments for the end of the year: oscillating reactions, electroplating, and diffusion-limited aggregation. Read more...
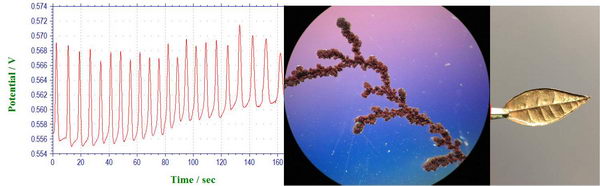
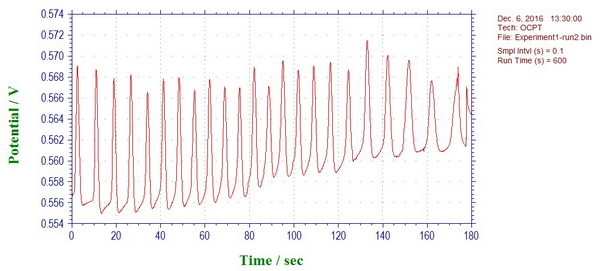
First, the quick experiment with Briggs-Rauschner oscillating reaction, during which we measure how the open circuit potential over time. Same as the color changes, the potential also oscillated regularly at about 10 seconds periods, since it also reflects the relative concentrations of the reagents in solution.
Of course, we had to try electroplating before finishing the course. 5 yen is easy to electroplate with copper sulfate solution:

I sacrificed some 50 yen coins for the next experiment:

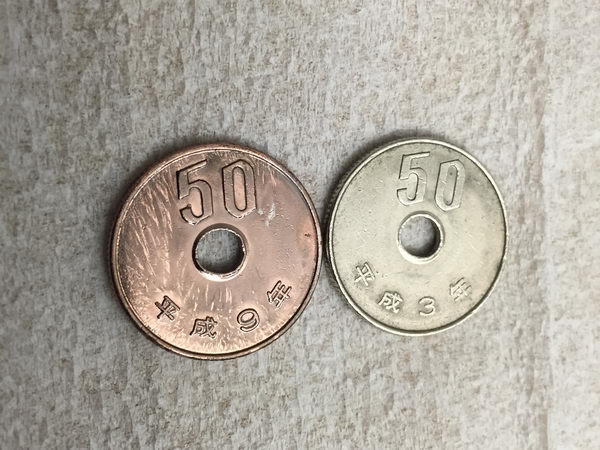
Then, it was time for more sophisticated objects:


Next, growing copper dendrites by diffusion-limited aggregation. More concentration solution:
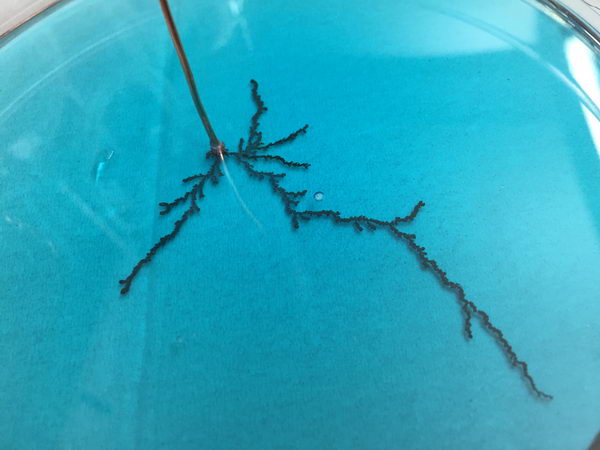
A more dilute solution gives a more fluffy-looking, denser structure:

Here is how the copper dendrite actually looks-like under a microscope: very much like an orange fir tree: