FY2021 Annual Report
Evolutionary Neurobiology Unit
Assistant Professor Hiroshi Watanabe

Abstract
Many of the research projects conducted in the Evolutionary Neurobiology Unit is aimed at elucidating the origins of animal systems and how key processes in their early evolution were established at the molecular and genetic levels. Specific targets of our research include the genetic and physiological features of neurons and other basic animal cells (glia, muscles, digestive cells, etc.) in the ancestral animals and early evolutionary processes of body axes and germ layers, which are fundamental to shape the multicellular animal individual.
These are biologically important issues, and although various hypotheses have been proposed over the years, there are still many unanswered questions. In our unit, we will unravel the secrets of the "birth of animals", that happened around 600 million years ago, by constructing a new systematic approach using state-of-the-art mass spectrometry, bioinformatics, and gene function analysis techniques for a group of primitive, and thus phylogenetically important, animal lineages (e.g., ctenophores and cnidarians). To further strengthen our study, we have started collaborations with marine biologists and AI researchers.
1. Staff
- Dr. Hiroshi Watanabe, Assistant Professor
- Dr. Eisuke Hayakawa, Group Leader
- Dr. Kurato Mohri, Staff Scientist
- Dr. Ryo Nakamura, Postdoctoral Scholar
- Dr. Hongdi Wang, Postdoctoral Scholar
- Dr. Ryotaro Nakamura, Research Unit Technician
- Dr. Yayoi Hongo, Research Unit Technician
- Chihiro Kawano, Research Unit Technician
- Kanako Hirata, Research Unit Technician (PoC)
- Akiko Tanimoto, Research Assistant
- Junko Higuchi, Research Assistant
- Mika Ogata, Research Assistant (PoC)
- Asami Kyan, Research Assistant (PoC)
- Ivan Mbogo, PhD Student
- Larisa Scheloukhova, PhD Student
- Osamu Horiguchi, PhD Student (JSPS DC1 fellow)
- Christine Guzman, PhD Student (JSPS DC1 fellow)
- Kun-Lung Li, PhD Student
- Minato Miyake, PhD Student
- Sen Hadife, PhD Student
- Chihiro Arasaki, Research Unit Administrator
Past Members
- Ms. Akiko Tanimoto, Research Assistant (February 2019–February 2022)
- Mr. Hibiki Fukunaga, Research Assistant (October 2019–March 2021)
- Ms. Rio Zakoh, Research Assistant (April 2018–March 2019)
- Dr. Mei-Fang Lin, Postdoctoral Scholar (May 2017–July 2020. currently Assistant Professor, National Sun Yat-sen University, Taiwan)
- Ms. Erina Kawai, Research Unit Technician ( August 2016–October 2019. Currently Research Unit Scientific Diver, OIST Marine Climate Change Unit)
- Dr. Amol Dahal, Research Unit Technician (May 2016–November 2018. Currently Lecturer, Kathmandu University)
- Dr. Shinya Komoto, Staff Scientist (October 2016–March 2018. Currently Research Support Specialist, OIST Scientific Imaging Section)
2. Collaborations
2.1 Identification of ion channel receptors of novel neuropeptides
- Type of collaboration: Joint research
- Researchers:
- Prof. Stefan Gruender, RWTH, Aachen University, Germany
2.2 Gene function analysis in the model ctenophoran Bolinopsis mikado
- Type of collaboration: Joint research
- Researchers:
- Prof. Kazuo Inaba and Assistant Prof. Kogiku Shiba, Shimoda Marine Research Center, University of Tsukuba, Shizuoka, Japan
2.3 Genome analysis of Zoantharian species
- Type of collaboration: Joint research
- Researchers:
- Assistant Prof. Mei-Fang Lin, National Sun Yat-sen University, Taiwan
- Associate Prof. James Reimer, University of the Ryukyus
2.4 AI-based peptide receptor prediction
- Type of collaboration: Joint researh
- Researchers:
- Dr. Honoo Satake & Dr. Akira Shiraishi, Bioorganic Research Institute, Suntory Foundation for Life Sciences, Kyoto, Japan
3. Activities and Findings
3.1 Metabolomic analysis of neurotransmitters in basal metazoans
Chemical neurotransmitters play essential roles in the bilaterian nervous systems. Some of primitive neurotransmitters/modulators, including neuropeptides, have been reported to be functioning in Cnidaria, the closest sister to all bilaterians, suggesting that the last common Cnidaria/Bilateria ancestor had already equipped with the chemical neurotransmission. However, the molecular repertory of neurotransmitters has been inconclusive in Cnidaria due to the difficulties in the precise molecular characterizations.
In this project, we are applying a modern analytical platform of LC-MS metabolomics to comprehensively characterize the neurotransmitter candidates in Cnidaria at the high molecular specificity. So far, we have found that the monoaminogenic molecules derived from L-aromatic amino acids found in Nematostella vectensis (Cnidaria) are different from those in bilaterian representatives (Fig. 1). We believe that our high-resolution metabolomic analysis of small chemical transmitters will provide a comprehensive and more reliable molecular catalogue of cnidarian chemical neurotransmitters and also a novel insight into the physiological nature of the primordial nerve systems.
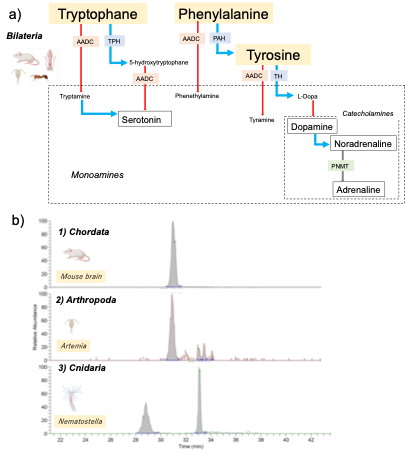
3.2 Anatomical and physiological studies of ctenophore nervous systems
The physiological feature (e.g. neurotransmitters) of neurons in the first animals remained unknown. Our peptidomics study identified a number of neuropeptides in the Ctenophora, which is one of the most basal animal lineages, and suggests that peptidergic neurotransmission is the ancestral feature of the nervous system. This year, we visualized neuropeptide-expressing neurons to examine anatomical features of the ctenophore nervous systems. We used the pelagic species Bolinopsis mikado (Fig. 2a), and the benthic species Vallicula multiformis (Fig. 2b) for this analysis. Immunohistochemical analysis of these peptides could identify many types of nervous structures in Ctenophora (Fig. 2c, d) In addition, we demonstrated that the synthetic mature peptides could induce behavioral responses such as muscle contraction.
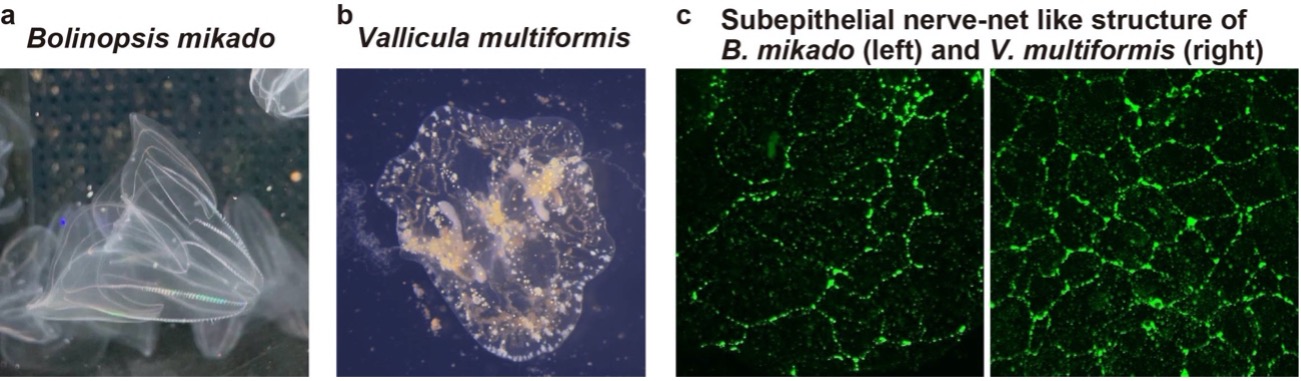
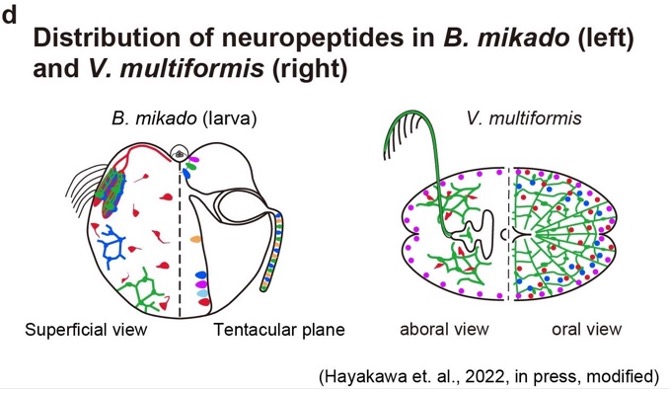 Figure 2. (a) Pelagic ctenophora B. mikado. (b) Benthic V. multiformis. (c) A similar subepithelial nerve-net structure was identified in B. mikado (left) and V. multiformis (right) by immunohistochemical analysis against a neuropeptide. (d) Schematic diagrams for comparison of peptides distribution between B. mikado (left) and V. multiformis (right). The same color indicates identical peptides.
Figure 2. (a) Pelagic ctenophora B. mikado. (b) Benthic V. multiformis. (c) A similar subepithelial nerve-net structure was identified in B. mikado (left) and V. multiformis (right) by immunohistochemical analysis against a neuropeptide. (d) Schematic diagrams for comparison of peptides distribution between B. mikado (left) and V. multiformis (right). The same color indicates identical peptides.
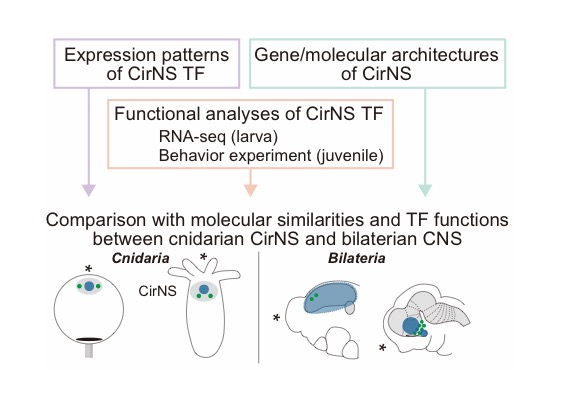
3.3 Evolution of the neural centralization
The evolution of the centralized nervous system (CNS) remains unknown. Most of the marine invertebrates develop a neuron assembly around oral/esophagus region (e.g. circumopharyngeal nervous system: CirNS), suggesting their pre-bilaterian origins. Previous studies have shown that expression patterns of bilaterian CNS-patterning genes exist in Cnidaria, however very little is known about the function of these homologs in cnidarian CirNS at the oral side. In FY2021 continuing from last year, further retrieving of our RNA-seq and data taken from single cell RNA-seq and oral expression were performed in order to decipher detailed molecular architecture of CirNS. In addition, new functional analyses using N.vectensis were performed to reveal the effect of CirNS related transcription factor (TF) on their behavior. By combining all our data from 2019 to 2021 (Figure 3), we obtained a new insight into the brain evolution focusing on the oral CirNS which seems also explaining why ancestral animals develop the neural assembly from diffuse nerve net.
4. Publications
4.1 Journals
-
Shikaya, Y., Takase, Y., Tadokoro, R., Nakamura, R., Inaba, M., & Takahashi, Y. Distribution Map of Peristaltic Waves in the Chicken Embryonic Gut Reveals Importance of Enteric Nervous System and Inter-Region Cross Talks Along the Gut Axis. Frontiers in Cell and Developmental Biology, doi: 10.3389/fcell.2022.827079 (Feb. 2022)
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
-
Hongo, Y., Collaborations with Analytical Chemistry, OIST-RIKEN Collaboration 1st Symposium: Green and blue planet -How can ecological research shape our future?, April 6–7, 2021, OIST (Invited Seminar, Oral)
-
Hongo, Y., and Watanabe, H., Evolution of Metabolism, OIST-RIKEN Collaboration 1st Symposium: Green and blue planet -How can ecological research shape our future? (Invited), April 6–7, 2021, OIST (Poster)
-
Hongo, Y., Watanabe, H., Konno, K., and Kazuma, K., Structure estimations of positional isomers using energy-resolved MSn, the 69th Annual Conference on Mass Spectrometry Japan, 2F-O10-1120 (Session Chair), May 20, 2021, Japan (Oral)
-
Hongo, Y., 質量分析で何が見えるのか?生命起源から進化まで覗いてみた, the 12th Okinawa Open Tech Seminar by OoPNet (invited), January 4, 2022, Okinawa (Oral)
-
Hongo, Y., Protein first-order structure originated by multi-phase heterogeneous reactions, the 17th Life in the Universe workshop by Astrobiology Center, February 10, 2022
5. Intellectual Property Rights and Other Specific Achievements
External Fundings
-
Grant-in-Aid for Scientific Research (C), Japan Society for the Promotion of Science, “Reconstruction of the ancestral neurons based on the common molecular signature of peptidergic nervous systems of early branching metazoans. (ペプチド作動性神経系に共通する分子シグネチャーに基づく祖先的神経細胞の再構築)” Lead PI: Watanabe H., Co-investigator: Hayakawa E. and Inaba K. (Shimoda), Amount 4.42 M Yen, Period: Apr. 2021 – Mar. 2023
-
Grant-in-Aid for Scientific Research, Ministry of Health, Labor and Welfare, ”Research on safety and risk communication for foods developed by new biotechnologies. (新たなバイオテクノロジーを用いて得られた食品の安全性確保とリスクコミュニケーションのための研究) H30-食品-一般-002)”, Lead PI: Kondo, K. (National Institute of Health Sciences)., Co-investigator: Hayakawa E., Amount 61.48 M Yen, Period: Apr. 2018 – Mar. 2022
-
Grant-in-Aid for Scientific Research (C), Japan Society for the Promotion of Science," Cross-species cross-linking proteomics to elucidate the evolutionary origin of synaptic protein complexes. (横断的クロスリンクプロテオミクスによるシナプスタンパク複合体の進化的起源の解明)” Lead PI: Hayakawa, E., Amount: 4.29M Yen, Period: Apr. 2019 – Mar. 2022
-
Grant-in-Aid for Scientific Research (C), Japan Society for the Promotion of Science, “Study for the reestablishment of positional information during regeneration of multicellular bodies of social amoebae. (細胞性粘菌多細胞体の再生過程における位置情報再構成機構の解析)” Lead PI: Mohri, K, Amount: 3.3 M Yen, Period: Apr. 2020 – Mar. 2023
6. Meetings and Events
Nothing to report
7. Other
Nothing to report.



