FY2022 Annual Report
Cell Proliferation and Gene Editing Unit
Assistant Professor Franz Meitinger

Abstract
Every day, millions of cells in our body divide to maintain essential tissue functions. Errors in cell division can lead to developmental disorders or cancer. The research of the unit is focused on molecular mechanisms of cell division and quality control in normal and cancer cells to understand tumor-suppressive mechanisms and identify biomarkers that confer a cancer-specific vulnerability to chemical drugs. The unit combines high throughput imaging, gene editing and genome wide screens to open new avenues for therapeutic development.
1. Staff
- Dr. Feng Ying Esther Ng, Postdoc
- Dr. Md Hazrat Belal, Postdoc
- Orie Arakawa, Technician
- Carmen Sparr, Technician
- Anna Pavlovska, Research Intern (from February 3, 2023)
- Mika Uehara, Research Unit Administrator (until October 31, 2022)
- Hitomi Ohtaki, Research Unit Administrator (from November 1, 2022)
2. Unit Opening
We started at OIST in August 2022. In the first few months we have managed to recruit excellent scientists who share our vision.
Our key technologies include high-throughput microscopy, gene editing, genetic screens and biochemistry.
We have started wet lab experiments and setting up genetic screens to identify cancer-specific mechanisms of cell division and proliferation.
3. Activities and Findings
Identification of a molecular mitotic stopwatch that exhibits transgenerational memory
Mitotic duration is tightly constrained, with extended mitotic duration being a characteristic of potentially problematic cells prone to chromosome missegregation and genomic instability. We show that memories of mitotic duration are integrated by a p53-based mitotic stopwatch pathway to exert tight control over proliferation. The stopwatch halts proliferation of the products of a single significantly extended mitosis or of successive modestly extended mitoses (Fig. 1). Time in mitosis is monitored via mitotic kinase-regulated assembly of stopwatch complexes that are transmitted to daughter cells. The stopwatch is inactivated in p53-mutant cancers, as well as in a significant proportion of p53-wildtype cancers, consistent with classification of stopwatch complex subunits as tumor suppressors. Stopwatch status additionally influences efficacy of anti-mitotic agents currently used or in development for cancer therapy.
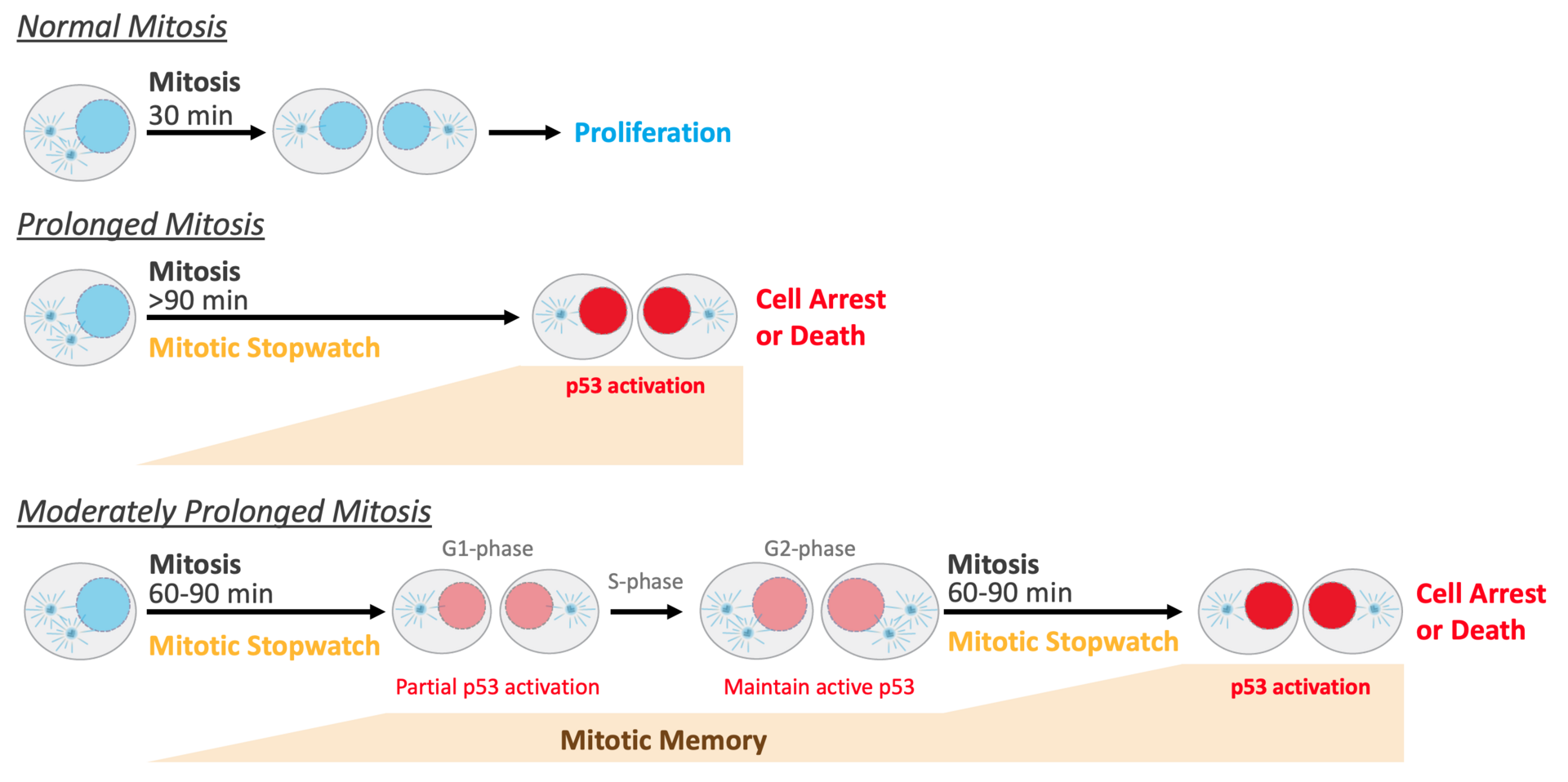
Figure 1: The Mitotic Stopwatch monitors the quality of cell division by measuring the duration of mitosis. Extended mitoses lead to cell arrest or death. (see text for more detailed explanation)
4. Publications
4.1 Preprint
- Meitinger, F., Davis, R.L., Martinez, M.B., Shiau, A.K., Oegema, K., Desai, A. (2022). Control of cell proliferation by memories of mitosis. bioRxiv 2022.11.14.515741; doi: https://doi.org/10.1101/2022.11.14.515741.
4.2 Oral Presentations
- Meitinger, F. ASCB/EMBO meeting, Washington DC, USA, Dec 4, 2022
- Meitinger, F. Ajou University School of Medicine (Korea) - OIST Joint Seminar, Okinawa, Japan, March 7, 2023
- Meitinger, F. OIST Internal Seminar, Okinawa, Japan, March 31, 2023



