N95-electrocharged filtration principle based face mask design using common materials
The content on this webpage is now out of date. Further work was undertaken after this webpage was created. The full details of this work can now be accessed from the published manuscript at: M. M. Bandi, "Electrocharged facepiece respirator fabrics using common materials", Proc. Roy. Soc. A. 476, 20200469 (2020).
Author: Mahesh M. Bandi, Nonlinear and Non-equilibrium Physics Unit, OIST Graduate University.
This is open-access information, made freely available for anyone to use.
Table of Contents
- N95 Filtration Principle
- Electrocharged Filtration
- Manufacturing Electrospun polymer fabric with Cotton Candy Machine
- Modified Cotton Candy Machine
- Basic Characterization of our homemade electrocharged polymer fabric
- Mask design based on the Cotton-Candy electrocharged polymer materials
- Final Details: IMPORTANT
- ACKNOWLEDGMENTS
The ongoing COVID-19 pandemic has necessitated use of face masks for protection. Many countries now mandate individuals to wear face masks when in public. However face masks have disappeared from store fronts, necessitating homemade cloth masks. But neither homemade cloth masks nor most commercial masks can filter the COVID-19 virion. The N95 mask presents the only available design capable of stopping the COVID-19 virion, because it contains an electrocharged layer capable of attracting and trapping aerosol droplets down to micrometer dimensions, virions, and bacteria in the 100s of nanometer dimensions. Unfortunately, this electrocharged polymer layer is manufactured through an industrially sophisticated electrospinning process that is a bit hard (but not impossible) to duplicate using commonly available materials.
This webpage details a process we have developed in-house at OIST to manufacture this electrocharged layer using commonly available parts and knowledge of basic electrical circuits to construct facemasks.
Our tests (detailed below) indicate that our face mask design outperforms homemade cloth masks and commercially available PM2.5 filtration masks and surgical masks, but they do not perform at the level of the N95 mask for several reasons explained below. This facemask design has not been tested or certified by any relevant authority such as NIOSH. This design is provided as-is with no guarantees.
N95 Filtration Principle
Face mask filtration mechanisms have to optimize between two competing requirements. The mask filters must have a pore size small enough to trap particles, but a pore size too small also prevents the individual from breathing, so mask filters cannot go below a certain average pore diameter. To meet this optimization requirement, N95 masks essentially operate on three principles (please see Figure 1 below for cartoon representation):
1) Inertial Impaction: Aerosol or dust particles typically 1 micron or larger in size with enough inertia to prevent them from flowing around the fibers in the filtration layers slam into the mask material and get filtered.
2) Diffusion: Particles smaller than 1 micron, usually 0.1 microns and smaller that are not subject to inertia undergo diffusion and get stuck to fibrous layers of the filter while undergoing brownian motion around the tortuous porous matrix of the filter fiber.
3) Electrostatic attraction: This mechanism employs electrocharged polymer or resin fibers that attract both large and small oppositely charged particles and trap them.
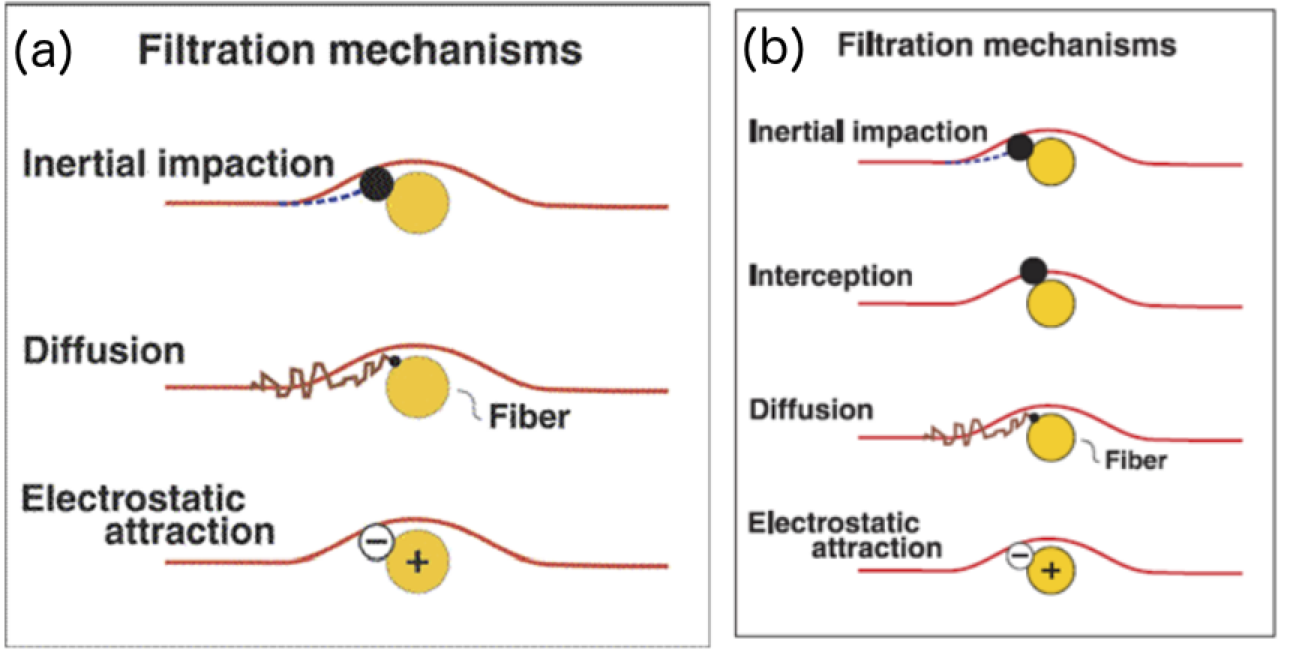
Figure 1: Cartoon representation of the three principles that N95 masks utilize. (a) The National Academies Press report from 2006 details three stages (Image credit: Institute of Medicine 2006. Reusability of Facemasks During an Influenza Pandemic: Facing the Flu. Washington, DC: The National Academies Press. https://doi.org/10.17226/11637.) whereas (b) the CDC webpage lists four principles, but we have subsumed interception principle listed by CDC within inertial impaction principle (Image credit: CDC webpage at https://blogs.cdc.gov/niosh-science-blog/2009/10/14/n95).
Any cloth or commercially available face mask can achieve the first and second principle with little effort. The third stage of electrostatic attraction is one of several design features that sets the N95 mask apart from other face masks. The ability to make one's own electrocharged filtration layer is not so straightforward.
Electrocharged Filtration
We know from common experience, especially in cold climates with low ambient humidity, that when two fabrics rub against each other they gain static electricity -- a phenomenon commonly known as triboelectric charging. Fabrics woven from natural fibers like wool or cotton, which possess higher roughness, and even synthetic fabrics like Nylon gain static charge from rubbing. The idea of exploiting charged fabrics to aid in filtration goes back a few decades (e.g. please see a brief review by Edward R. Frederick (1974) Some Effects of Electrostatic Charges In Fabric Filtration, Journal of Air Pollution Control Association, 23. 1164. DOI: 10.1080/00022470.1974.10470030), and indeed some early face mask designs incorporating electrocharged filtration did use wool or felt fibers. Over time though, electrospun polymer fabrics have become the mainstay material for the electrocharged layers in face mask designs.
The electrospun polymer material is manufactured using a method called electrospinning -- a widely used platform for generating polymer nanofibers. In this method, molten polymer of high viscosity is forced out of tiny orifices. The metallic container (we will call it the emitter) with the orifices holding the polymer melt is positively charged and a flat plate or drum placed at a distance is negatively charged (we will call this the collector). As the molten polymer is forced out of the emitter's orifice, the positively charged polymer melt shoots out and is attracted towards the negatively charged collector, and rapidly cools down over the distance covered between the emitter and collector, thereby resulting in polymer nanofibers that are electrically charged. This industrial electrospinning process is not generally found in everyday use.
There are many youtube videos out there that demonstrate the electrospinning process in both lab-based and industrial settings. We include a few representative links to motivate your intuition here. They were the first hits we got on youtube, otherwise there's no specific reason why we chose these links over any other(s). CAUTION: We do not endorse any industrial or academic lab's electrospinning process.:
https://www.youtube.com/watch?v=hlHUVJkDuNo
https://www.youtube.com/watch?v=Dn6r1Ag1npE
https://www.youtube.com/watch?v=x0VFeMTSIb4
https://www.youtube.com/watch?v=PJWMtdaYxi8
https://www.youtube.com/watch?v=6hRv7m1MOC0
https://www.youtube.com/watch?v=OIUSSUWhXhM
A cartoon illustration of the melt polymer electrospinning principle is shown below in Figure 2.
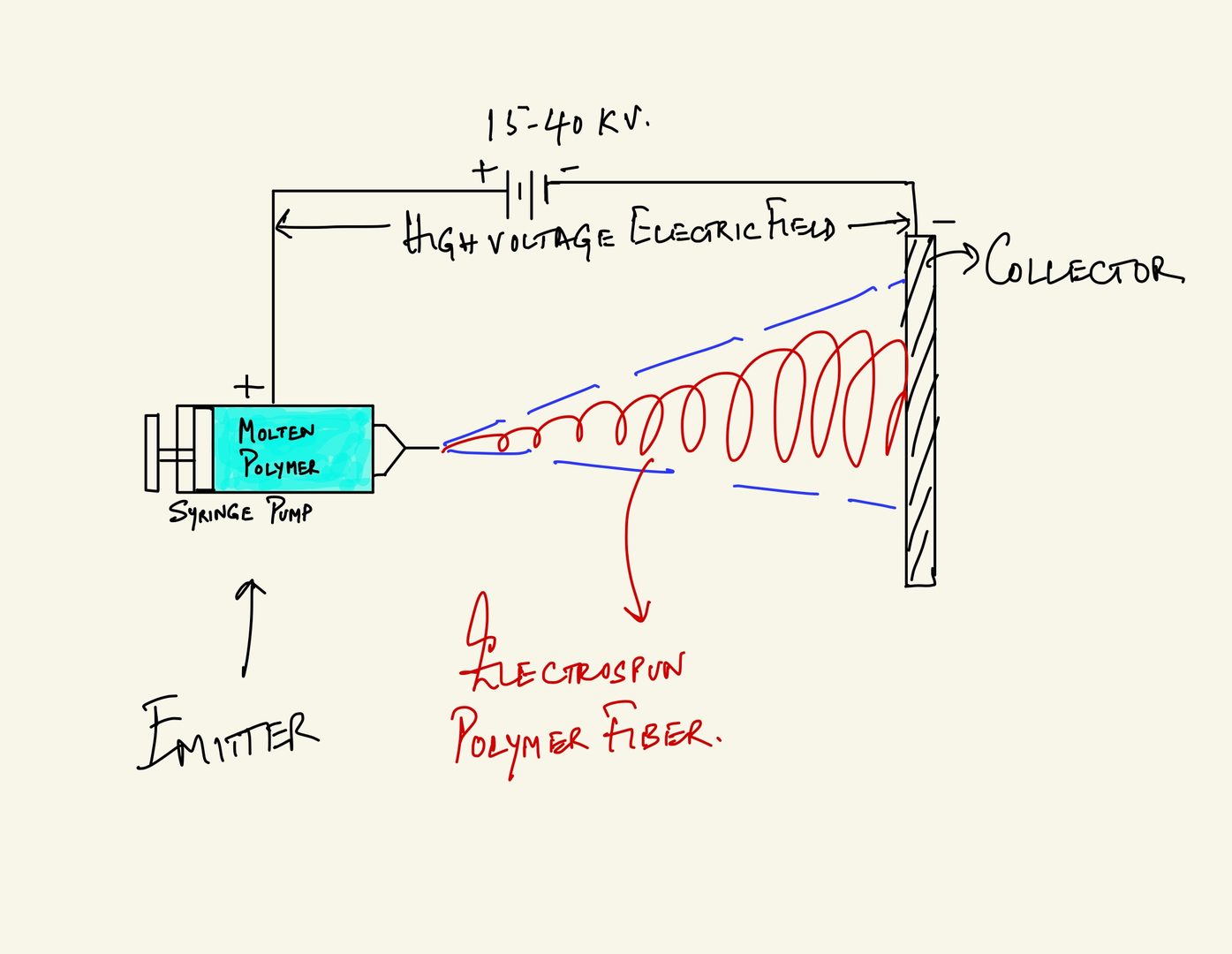
Figure 2: Cartoon illustration of the electrspun polymer nanofiber manufacturing mechanism. Molten polymer in a viscous fluid form is forced through an orifice, usually a motorized syringe pump, shown as the EMITTER. A distance apart from the orifice is a metallic plate, we denote as the COLLECTOR. The syringe pump's metallic needle is connected to the positive, and collector is connected the negative terminal of a high voltage source, usually in the order of 10s of kilovolts. As the viscous molten polymer is forced out of the syringe needle, the DC electric field between the emitter and collector forces the droplet to stretch, undergoing extension and cooling simultaneously, and gets deposited as polymer nanofiber (shown as a red spiral in the cartoon illustration) on the collector plate. This fast extending and cooling polymer gets spit out as shown bounded within the blue dashed line in the illustration.
Manufacturing Electrospun polymer fabric with Cotton Candy Machine
A simple way to generate electrospun polymer fabric with commonly available parts is by slightly modifying a cotton candy machine. We all know the basic principle on which a cotton candy machine operates:
1) There is a drum, at the center of which sits a fast spinning container holding 3 parts sugar and 1 part water. This container, which we call the EMITTER, is heated to about 160 - 175 degrees centigrade to caramelize the sugar into a viscous fluid.
2) The fast spinning container has small holes through which the viscous caramelized sugar flows out and the centrifugal force extends the viscous sugar droplet into a thin nanocrystalline fiber of sugar, which we collect with a stick in the form of cotton candy.
3) Now, let's modify this slightly. The electrospinning system requires a high voltage DC electric field to accelerate the polymer droplet from collector to emitter. The cotton candy machine instead provides mechanical acceleration from the centrifugal spinning of the emitter, so it does not require such a high voltage. But it does still require a DC electric field to induce charges in the polymer nano fabric. Let's say we setup a low voltage DC electric field (we worked in the range of 12 - 24 volts) using a lab voltage source-- you can also use a car battery (please see cartoon illustration in figure 3 below).
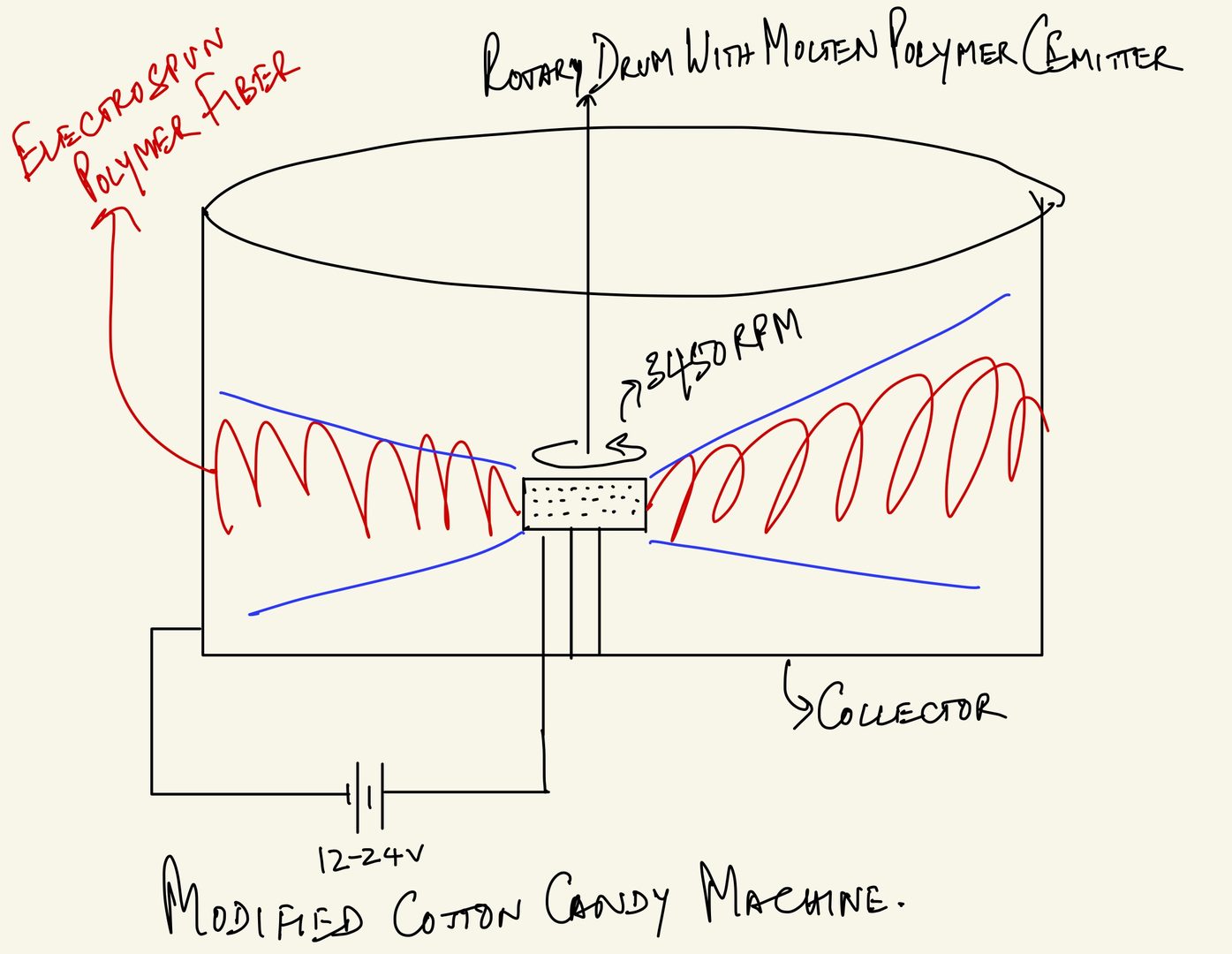
Figure 3: Cartoon illustration of a cotton candy machine comprised of a large cylindrical drum (called the COLLECTOR), at the center of which is a heated container (which we call EMITTER) in which we typically pour moist sugar (3 parts of sugar with 1 part of water + food coloring if you wish). The container spins at seveal 1000 RPM and as the viscous caramelized sugar fluid oozes out of the holes in the EMITTER, and the centrifugal force splashes the caramelized sugar fluid droplets, and in the process, extending them into thin crystalline nanofibers. Applying a low electric field (12-24 V) between the emitter and collector, and replacing sugar with molten polymer, provides a high throughput electrospinning system one can construct at one's home with simple parts.
4) And instead of sugar, we pour powdered polypropylene polymer -- you can take any of your polypropylene plastic bottles, cut them up in pieces and put them in a high power blender to get powdered polypropylene -- and start spinning at several 1000 RPM. You now start generating the electrospun polymer which starts collecting as a fabric comprised of electrocharged nanofibers on the collector surface.
CAUTION: The melting and extrusion process of polypropylene at 160C (or other plastic materials) would likely inactivate most biological material (in particular bacteria, fungi and viruses). However, autoclaves are typically run at comparable temperatures (160- 190C) on a dry cycle for 15-120min. For compatible materials, sterilization is usually done in a wet cycle at 120C and fairly high pressure (something like 100 kPa). The steam helps to break open cells and irreversibly denature protein and nucleic acids. Point being, quickly melting polymer powder of contaminated bottles passed through a blender may not result in destruction of 100% of the infectious properties of potential contaminants. This is of particular concern when using the resulting material for face protection. We therefore advise throwing the plastic containers in a pressure cooker and cook in high steam for 20 minutes or so before throwing them in a food processor to turn into powdery material.
5) We used polypropylene for a simple reason. The standard cotton candy machine available commercially has a heating temperature between 160 - 175 degrees centigrade. Polypropylene has a melting point temperature of 160 degrees centigrade. So the cotton candy machine needs no modification for the heating element. Otherwise, one is free to use other plastic long-chain polymeric materials like Polystyrene or Polyethylene (PET bottles, for instance). Some of the parameters such as melting point and applied electric field will certainly vary with the material used, but its easily figured out by quick trial-and-error, it ain't rocket science.
6) An easier way would be to dissolve the polyproylene in an organic solvent (e.g. Acetone), but we figured acetone is not a commonly available material.
7) Additionally, we were fortunate to have a plastic cover that we could place on our cotton candy machine. We simply drilled a hole in it and stuck a pipe through it connected to an ULVAC vacuum pump to evacuate the chamber -- we also placed silly putty or bread dough to seal the lid and container during evacuation. We did this simply to keep the environment clean and free of dust particulates during the electrospinning process in our rotary jet electrospinning system -- that's the fancy name we decided to give our in-house electrospinning system rigged from common parts. We realize, most people don't have access to a vacuum pump, at least not in residential environments, but if you're at a university lab that's still open (our's is), you can use this simple hack to make sure your electrospun polymer nanofibers are manufactured in semi-clean room conditions. In any event, the vaccum pump is not necessary, but helps. Please see the figure 4 below for a simple cartoon illustration.
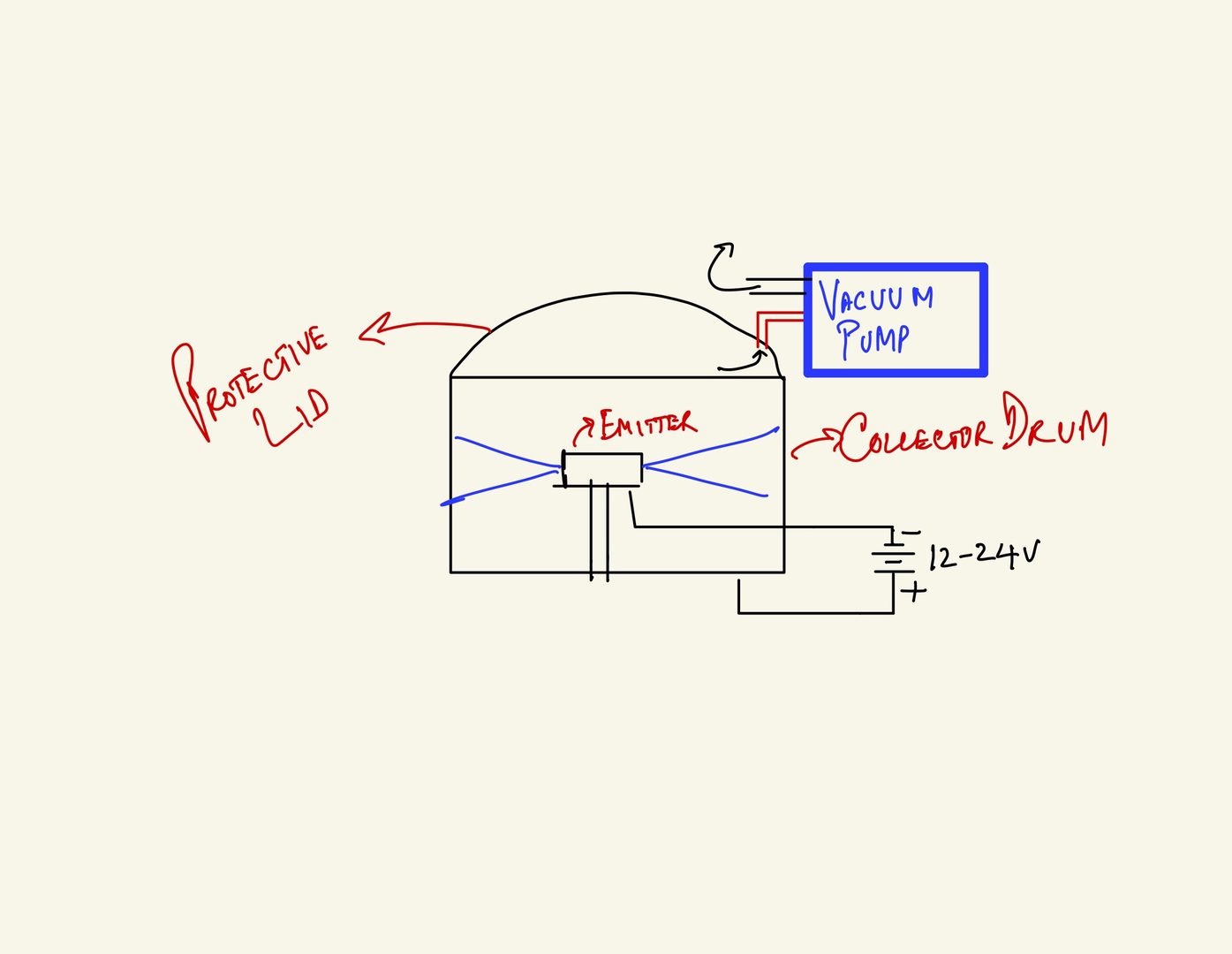
Figure 4: Cartoon schematic of modified cotton candy machine with (non-essential) vacuum pump attachment.
7) Also, if you don't have a cotton candy machine, there are several DIY videos to rig your own setup with minimal, commonly available parts. We include a few DIY youtube video links here. Once again, we included links that were immediate hits on youtube and there was no specific reason to choose these over others. And once again, CAUTION: We do not endorse any particular DIY cotton candy method:
https://www.youtube.com/watch?v=KN7Ac5Le434
https://www.youtube.com/watch?v=04oBgUyIr8I
https://www.youtube.com/watch?v=qYubkFzl0F8
https://www.youtube.com/watch?v=kk_2O8w1Uec
https://www.youtube.com/watch?v=7KpXkrtwc_Q
https://www.youtube.com/watch?v=s7E28_sJoEI
https://www.youtube.com/watch?v=7Dn47kO2p6E
Modified Cotton Candy Machine
We were lucky to locate a large cotton candy machine used in professional settings (Paragon Classic Cotton Candy Machine: https://www.amazon.com/Paragon-Classic-Floss-Cotton-Machine/dp/B005C9GT5O/ref=sr_1_1?dchild=1&keywords=Paragon+Classic+Floss+Cotton+Candy+Machine&qid=1586409734&sr=8-1 ). We had to insulate the emitter and collector with a thick teflon disk to avoid shortcircuit of the high voltage electric field source, but otherwise, we were pretty much in business very quickly, once we made the minor modification to include the high voltage connections.
Note: The electrospinning process uses high DC voltage to accelerate the polymer droplet from emitter to collector. The rotary jet spinning method on which the modified cotton candy machine is based uses mechanical acceleration via centrifigual spinning. We initially did not pay attention to this tiny, yet important detail and realized our modified candy machine would heat up and re-melt the polymer nanofiber fabric after it was deposited on the collector drum. Going back and studying the literature, we realized our mistake and were able to get away with 12 - 24 V in DC. The very basic characterization we present below is after fixing this error.
We used polypropylene powder for our base material, and quickly got electrospun polymer nanofibers at much higher throughput than the traditional syringe injection electrospinning process. We used a few 10s of grams of polypropylene material per run, and the resultant electrospun polymer nanofiber material would collect on the inner wall of the drum (the COLLECTOR), which we simply cut with scissors and sandwiched between two thick glass plates to turn into a porous fabric sheet.
Basic Characterization of our homemade electrocharged polymer fabric
We ran some characterization tests on our electrospun polymer nanofiber fabric. Details below:
1) Shown below are scanning electron micrographs for two samples, one is from our internal OIST-prepared sample and the other is from a commercial N95 mask. We see no structural difference, and the filaments we produce with the modified cotton candy machine go down to 10s of nanometers just like the commercial one. No, we're not telling you which is which, go figure out for yourselves. The trained eye just might manage to distinguish the OIST-internal from commercial-grade N95 SEM at the 50 micron and 20 micron scale, but otherwise, between figure 5 - 9 below, the untrained eye cannot distinguish one sample from the other.
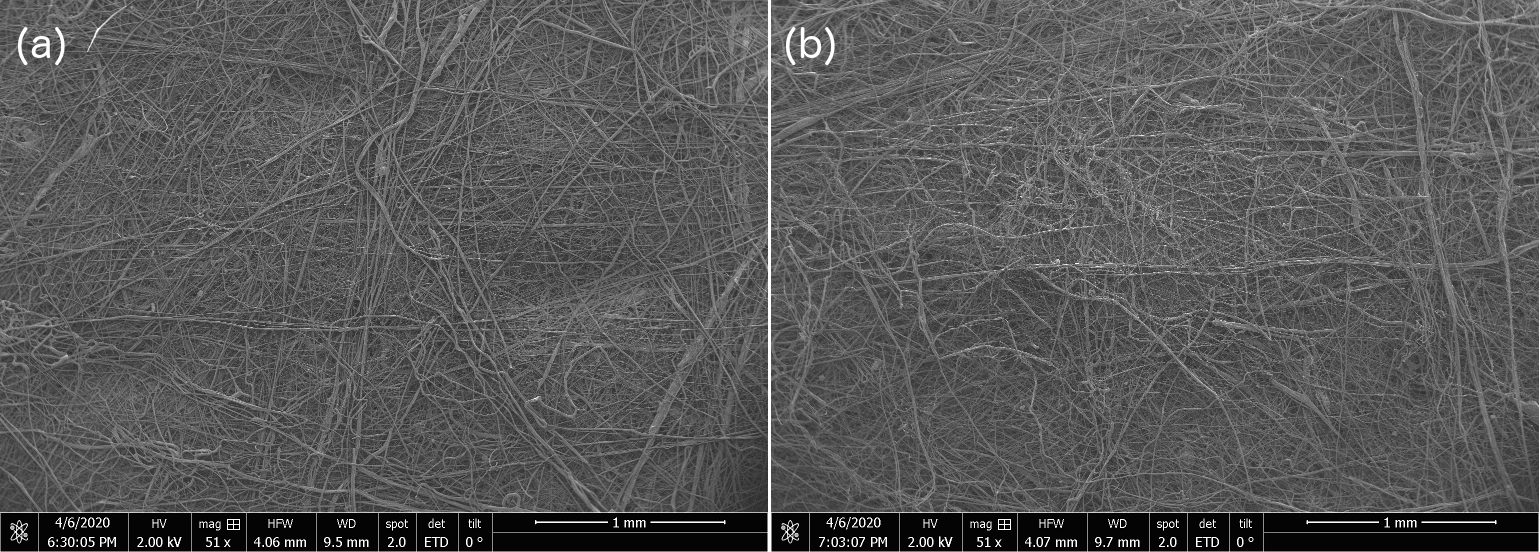
Figure 5: Scanning Electron Microscopy (SEM) Micrograph images for OIST-made and commercial N-95 electrocharged layer sample at 1 mm resolution shows no discernible structural differences between the two samples. We are not specifying which of (a & b) are OIST-internal versus commercial N95 sample SEMs.
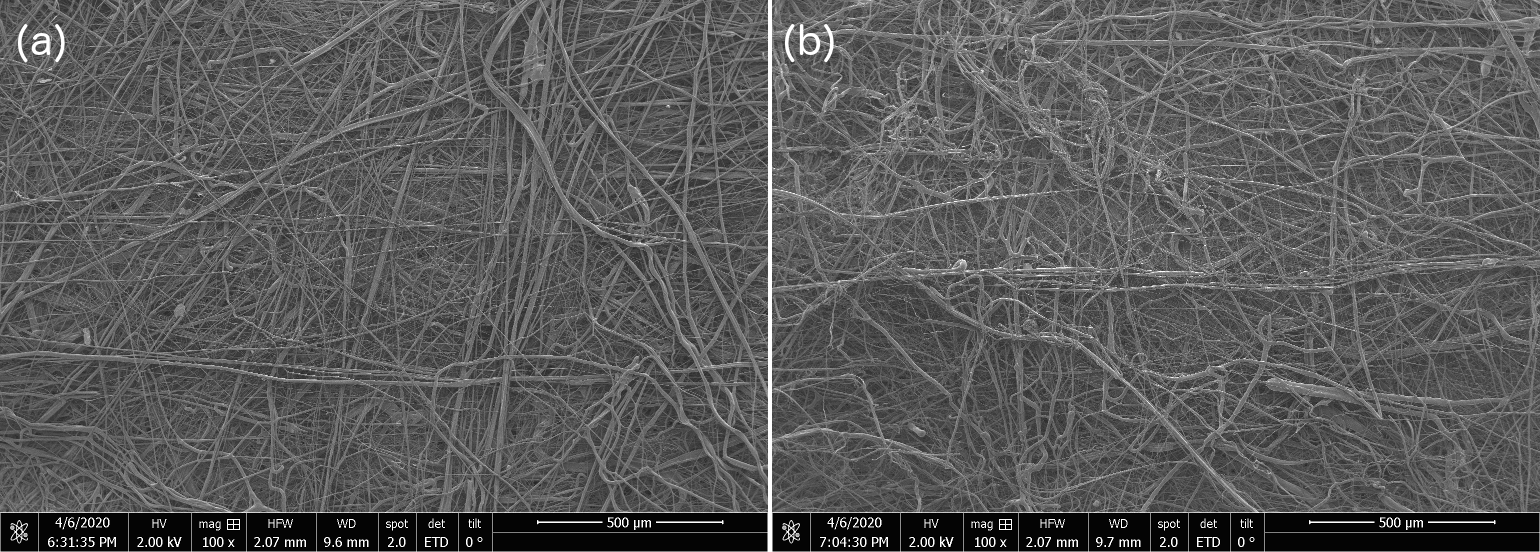 Figure 6: Scanning Electron Microscopy (SEM) Micrograph images for OIST-made and commercial N-95 electrocharged layer sample at 500 micron resolution shows no discernible structural differences between the two samples. We are not specifying which of (a & b) are OIST-internal versus commercial N95 sample SEMs.
Figure 6: Scanning Electron Microscopy (SEM) Micrograph images for OIST-made and commercial N-95 electrocharged layer sample at 500 micron resolution shows no discernible structural differences between the two samples. We are not specifying which of (a & b) are OIST-internal versus commercial N95 sample SEMs.
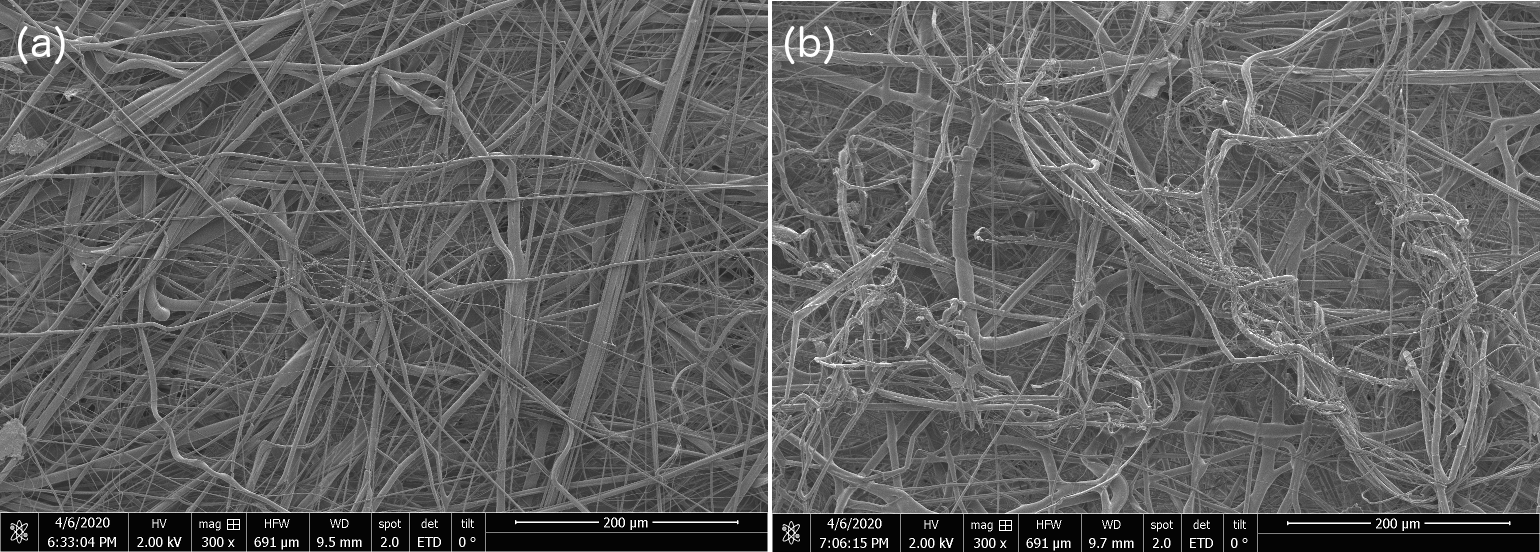
Figure 7: Scanning Electron Microscopy (SEM) Micrograph images for OIST-made and commercial N-95 electrocharged layer sample at 200 micron resolution shows no discernible structural differences between the two samples. We are not specifying which of (a & b) are OIST-internal versus commercial N95 sample SEMs.
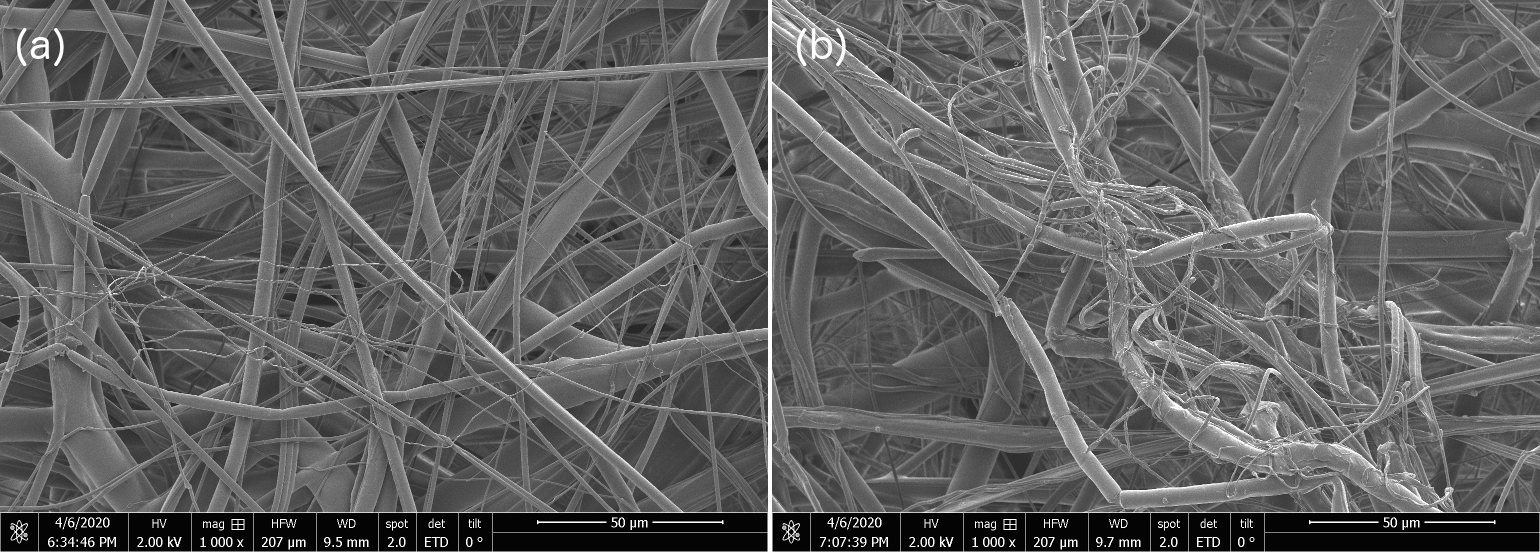
Figure 8: Scanning Electron Microscopy (SEM) Micrograph images for OIST-made and commercial N-95 electrocharged layer sample at 50 micron resolution shows no discernible structural differences between the two samples. We are not specifying which of (a & b) are OIST-internal versus commercial N95 sample SEMs.
 Figure 9: Scanning Electron Microscopy (SEM) Micrograph images for OIST-made and commercial N-95 electrocharged layer sample at 20 micron resolution shows no discernible structural differences between the two samples. We are not specifying which of (a & b) are OIST-internal versus commercial N95 sample SEMs.
Figure 9: Scanning Electron Microscopy (SEM) Micrograph images for OIST-made and commercial N-95 electrocharged layer sample at 20 micron resolution shows no discernible structural differences between the two samples. We are not specifying which of (a & b) are OIST-internal versus commercial N95 sample SEMs.
2) We shot 50 nanometer fluorescent colloidal particles in an airstream at our material at 30 liters/min (air speed for normal respiration) and 85 liters/min (air speed for increased respiration). The material traps these particles as confirmed on a confocal microscope down to 500 micron thickness of the electrospun, electrocharged polymer fabric. As a comparison, the COVID-19 virion is estimated to have a diameter of anywhere between 50 - 200 nanometers. Some recent publications we obtained these numbers from for the COVID-19 virion dimensions are cited below for your reference:
70 - 90 nm diameter according to this source: Jeong-Min Kim et al. Identification of Coronavirus Isolated from a Patient in Korea with COVID-19, Osong Public Health Res. Perspect. 11, 3-7 (2020).
50 - 200 nm diameter according to this source: Nanshan Chen et al. Epedimiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, , 395, 5017-13 (2020).
Ergo, our 50 nanometer colloidal particles fall at the lower bound of the estimated diameter of the COVID0-19 virion.
The question then is, does the COVID-19 virion (with an estimated diameter between 50-200 nanometers) have any surface electric charge that permits it to get attracted to the electrocharged polymer layer, whether it is internally OIST-produced or commercially produced by N95 facemask manufacturers? The answer to this question comes below from Prof. Matthias Wolf of the Molecular Cryo-Electron Microscopy Unit, OIST Graduate University:
The net charge would be determined by the sum of the charges exposed on the surface. This can be calculated from the protein structure(s) at the surface.
However, if we want to catch the virus with charged filters, there are two small issues:
- The virus is not airborne, as far as we know. Like most enveloped viruses, it's hydrated or in solution - in fact, it is transmitted by aerosol droplets, and when it gets dehydrated, its lipid membrane collapses and its proteins get denatured (within a certain time, depending on temperature etc), in other words, it is rendered inactive.
- The net charge of a protein in solution (water, typically) depends on the pH. Every amino acid has a pKa (the log of the acid dissociation constant), which is an equilibrium constant indicating the pH at which the charges are balanced (net neutral). There are pKa values for each chemical group. If the pH is lower than the pKa, that amino acid becomes protonated (if it can). At low pH, there is an abundance of positively charged protons. Even some acids can become protonated at low pH.
One can approximate the net charge of a linear peptide from its sequence and by providing a pH for the solvent using tools on the web - e.g. http://protcalc.sourceforge.net/. However, this does not take into account that structures are folded in 3D and some charges are hidden inside. Although it is only a rough value, it's ballpark-wise okay, because residues buried inside are often hydrophobic (ie. not charged).
There are several tools available online to calculate net charges of folded protein structures as well as their surface charge distribution, e.g. DELPHI http://honig.c2b2.columbia.edu/delphi
The isoelectric point pI is the pH at which the net charge is neutral. Below the pI, the net charge is positive, above it's negative. The reference by B. Michen and T. Graule, "Isoelectric points of viruses", J. Appl. Microbiology 109, 388-397 (2010) lists some examples of viruses - Most of their pIs are <7, suggesting that most viruses would be net negatively charged at neutral pH.
One would think that the COVID-19 virion is also net negative. The main surface protein is the spike protein (S) e.g. http://www.rcsb.org/structure/6VXX. It is glycosylated, thus has aome amino acids (arginines, mostly) chemically attached to sugars that can be charged, like sialic acid - these would also contribute to an overall negative charge.
When we input the sequence of the spike 6VXX into http://isoelectric.org/calculate.php, it comes up with a pI=5.8, thus net negative.
3) We also tested the decay in static electricity of the electrocharged fiber under humid conditions (due to exhalation) -- the electrocharged layer decays in over 5 hours.
Mask design based on the Cotton-Candy electrocharged polymer materials
Once the electrocharged polymer nanofiber material is at hand, rest of the process is quite straightforward, and there are several ways to construct a mask from this material. In our case, we followed the procedure below:
1) We produced two batches of electrospun polymer fabric at zero electric field to serve as our outer fabric material, for every 3 batches of electrospun polymer fiber at low DC electric field 12 - 24 V DC.
2) Each batch of the electrospun polymer fiber was cut with an ordinary (but clean and disinfected) scissors from the inner surface of the COLLECTOR, which yielded a rectangular sheet.
3) This sheet was pressed between two clean and disinfected 1 cm thick glass plates we had in the laboratory to obtain the fabric we worked with. Any two clean and flat surfaces that apply heavy load from above to flatten the fabric will work.
4) The material was then laid out in 5 layers as follows:
- Layer 1: Zero electric field polymer fabric.
- Layer 2-4: 12 - 24V DC electric field polymer fabric.
- Layer 5: zero electric field polymer fabric.
5) The layered material was then cut in square pieces of 6 cm x 6 cm. We then folded one dimension of this square layered material at the 2cm and 4 cm points to create a 5-layered sheet of dimensions 3.5 cm x 6 cm like a commercial facemask shown below and then stitched together:
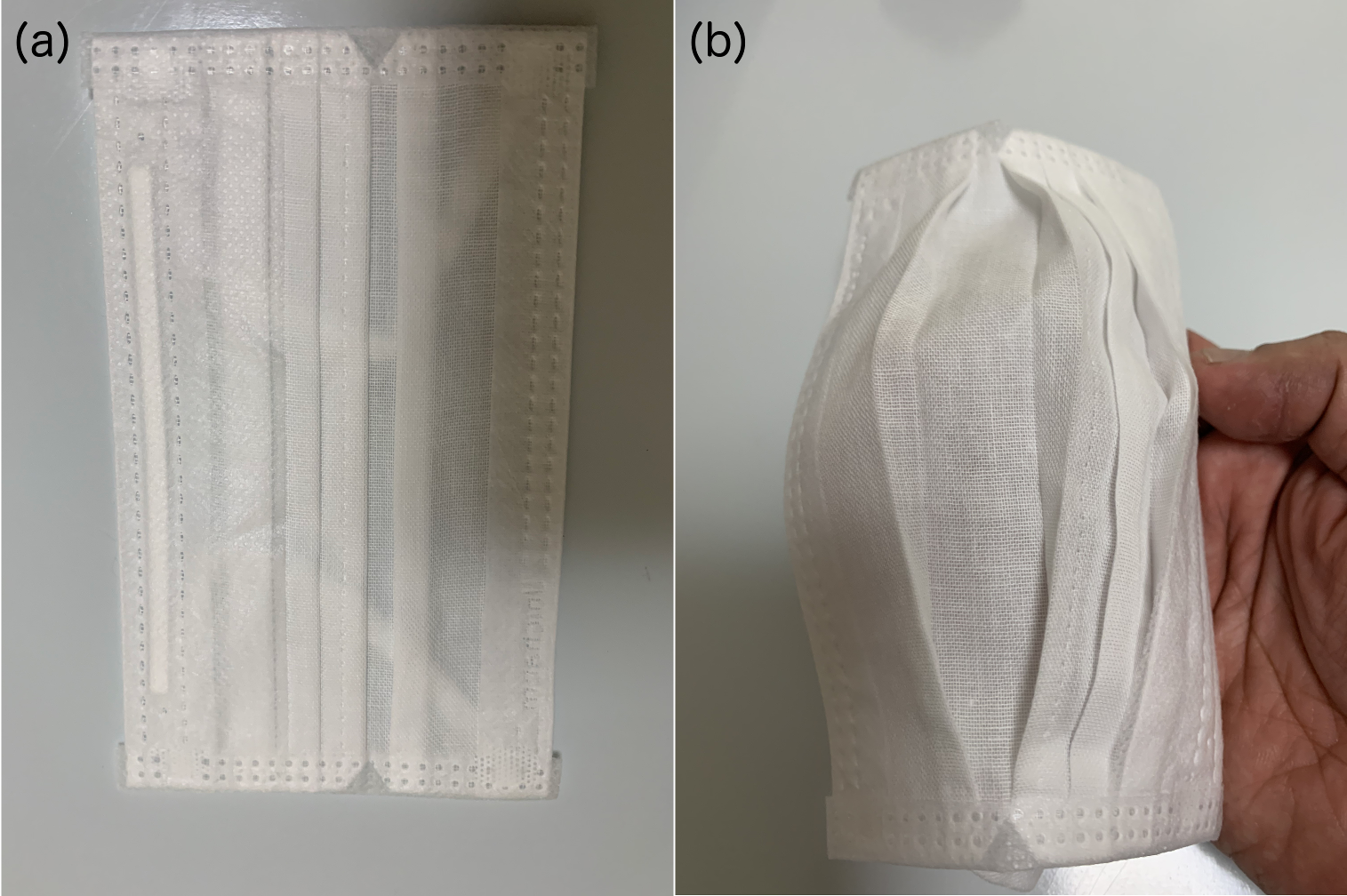
Figure 10: (a) A commercial fabric mask with folds and which expands when worn on the face to reveal the folds as shown (b) here by intentionally expanding with the hand.
But a far more elegant and easy to implement design that does not involve stitching comes from Farhad Manjoo and is available online from the New York Times with step-by-step instructions. Farhad's design with our layered material would work just as well: https://www.nytimes.com/2020/03/31/opinion/coronavirus-n95-mask.html
Final Details: IMPORTANT
We now provide an enumerated list of what our facemask design can and cannot achieve:
1) Based on our very preliminary and quick characterization, we know our electrocharged polymer nanofiber fabric manufactured internally at OIST can stop colloidal particles down to 50 nanometer diameter. That suggests, it may well offer protection against micro-aerosol droplets, bacteria and viruses. However, we have not tested our material against bacteria or viruses.
2) Based on humidity tests, we know the electrocharged layer we made in-house degrades due to humidity, be it ambient humid conditions or simple exhalation in about 5 hours. Our design is therefore only good for use-and-throw.
3) The commercial NIOSH certified N95 masks come with several superlative design features that our simple mask design cannot offer. First, NIOSH tested N95 masks are especially designed for fit, so no air escapes in or out from the gap between mask perimeter and skin. Our mask design definitely has gaps, and cannot meet that criterion. That extra design feature available in NIOSH certified N95s requires further engineering that we neither have bandwidth nor time to delve into.
4) NIOSH certified N95 masks also incorporate another elegant (depending on who you talk to) feature. An exhalation valve, which shuts off when the individual is breathing, so air is breathed in through the filtration layers, but exhaled air goes out the valve, so the electrocharged layer does not degrade under humidity. We put the caveat -- depending on who you talk to -- because if an infected individual wears the N95 mask, the exhalation valve does not protect neighboring individuals from catching the virion. Has anybody thought of this at all?
IN CONCLUSION: Our simple home-hacked design for electrocharged polymer nanofiber fabric and simple mask design borrowed from available designs, outperforms commercially available PM2.5 masks like the one shown in Figure 10 and surgical masks because our design has an electrocharged filtration layer while they do not. On the other hand, our design lacks some of the crucial design features that the N95 mask possesses, such as fit and exhalation valve.
ACKNOWLEDGMENTS
We thank Ms. Noriko Ishizu and the Nanofab team at OIST for help with Scanning Electron Microscopy, OIST Imaging Section for help with Confocal Microscopy, and Mr. Micheal Cooper and Chris Wu from the OIST Digital Services Section for real-time help with webpage fixes while editing was in progress, thereby allowing us to construct the full webpage in under 4 hours.
We used several references that we did not have time to cite here, and we sincerely apologize for it. All the references we have studied in the run-up to this project are available from my google drive folder for your use. They can be accessed from this link: https://drive.google.com/open?id=1j1aeymp2H7sAelLau9RHqMYnGGAZO032



