FY2021 Annual Report
FY2021 Annual Report
Molecular Neuroscience Unit
Assistant Professor Marco Terenzio

- (From left to right) Lower row: Maria Fransiska Emily, Sarah Zakiah, Lokesh Agrawal, Miki Otsuki. Upper row: Madeleine Le Coz Sara, Sandra De La Fuente, Emad El-Agamy Abdelaal, Laurent Guillaud, Marco Terenzio.
Abstract
In 2021 we have continued the buildup of the laboratory and published our first research paper, which studies the effect of 3D geometry in facilitating axonal vectorial growth. We have also developed additional fibrous scaffolds using biodegradable and biocompatible compounds, which we have filed a patent for. On the biological side, we continued our investigation of both axonal translation after injury and the mechanism underlying axonal degeneration in ALS using human iPSC.
1. Staff
- Assistant Professor Marco Terenzio, Unit Leader
- Dr. Laurent Guillaud, Group Leader
- Dr. Lokesh Agrawal, Post-doctoral Scholar
- Dr. Madeleine Le Coz, Post-doctoral Scholar
- Dr. Sandra De La Fuente, Post-doctoral Scholar
- Sara Emad El-Agamy Abdelaal, Ph.D. Student
- Maria Fransiska Emily, Ph.D. Student
- Sarah Zakiah, Ph.D. Student
- Maria Fernanda Bolanos Alejos, Ph.D. Student
- Maria Paglione, JSPS intern (December 2021-March 2022)
- Akiko Guzman, Research Unit Administrator
2. Collaborations
2.1 Local Translation and axonal dysfunction in Amyotrophic Lateral Sclerosis
- Type of collaboration: Joint research
- Researchers:
- Professor Andrea Malaspina, UCL London, UK
2.2 Local axonal and synaptic maintenance mechanisms in a Drosophila model of Wallerian degeneration
- Type of collaboration: Joint research
- Researchers:
- Assistant Professor Lukas Neukomm, University of Lausanne, Switzerland
- Maria Paglione, Ph.D. student
3. Activities and Findings
Neurons are highly polarized cells with an elongated axon that extends far away from the cell body. To maintain neuronal homeostasis, neurons rely extensively on axonal transport of membranous organelles and other molecular complexes in addition to local translation of proteins. Axonal transport plays a central role in the establishment of neuronal polarity, axonal growth and stabilization and synapses formation, allowing for precise spatio-temporal activation and modulation of numerous molecular cascades. Anterograde and retrograde axonal transport is supported by various molecular motors, such as kinesins and dyneins, and a complex microtubule network. We have been focusing on the interactors of a Dynein subunit, which is critical for the survival of sensory neurons (Terenzio et al., 2020). To this extent we created several fusion constructs of DYNLRB1 and a promiscuous biotinylating enzyme to selectively label DYNLRB1’s interactors and identify them by mass spectrometry. We heave infected DRG neurons with our fusion constructs and performed a proteomic screen to identify DYNLRB1 interactors. We are now in the process of characterizing the most promising candidates in vitro and in vivo and their potential implication in the genesis of neurological disorders.
We are also focused on strategies to promote axonal regeneration through to use of specialized substrates and the tools we have developed to investigate the mechanisms underlying axonal degeneration. We investigate what drives the rate of progression of neurodegeneration looking at a paradigm of fatal neurodegenerative disorders: Amyotrophic Lateral Sclerosis (ALS). To overcome the limitations of murine models, we directly derive motor neurons (MNs) from ALS patient’s fibroblasts to assess how local mechanisms of protein production (translation) is changed in motor cells from ALS patients. Last year we established a differentiation protocol for human pluripotent stem cells (iPSC) into MNs. In 2021 we have continued to characterize the hallmark of disease progression in these MNs and collect samples. We will perform a proteomic and transcriptomic analysis this coming year to better elucidate the mechanisms behind neurodegeneration in human MNs.
Finally, we aimed to deploy fibrous 3D scaffold/implants for the directed growth of axons, to restore and repair lost neuronal connections. We took advantage of state-of-the-art 2-photon photolithography to fabricate highly ordered and biocompatible 3D nanogrid structures to enhance neuronal directional growth. We characterized the physical and chemical properties and proved the biocompatibility of said scaffolds by successfully culturing primary sensory and motor neurons on their surface. Axons could grow in 3D between different layers of the scaffold and extended along the fibers with a high degree of alignment to the pattern of the nanogrid, as opposed to the lack of directionality observed on flat glass or polymeric surfaces (Figure 1). Thus, our findings provide a proof of concept and explore the possibility of deploying aligned fibrous 3D scaffold/implants for the directed growth of axons and could be used in the design of scaffolds targeted towards the restoration and repair of damaged nervous tissue (Agrawal et al., 2021).
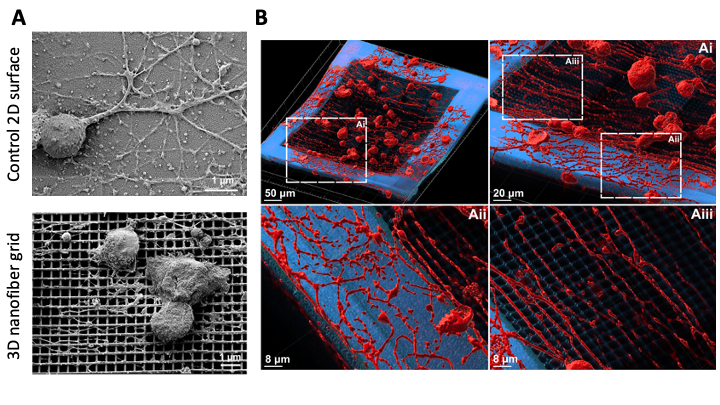
Figure 1: electromicroscopy (E.M.) images of adult DRG neurons growing on a 2D and 3D nanofiber scaffold (A). Immunofluorescence analysis of the 3D nano-grid. DRG neurons grow with an arborized morphology on the 2D control surfaces of the grid but present an aligned modality of growth on the fibers. Tubulin staining in red (B).
4. Publications
4.1 Journals
- Development of 3D culture scaffolds for directional neuronal growth using 2-photon lithography. L. Agrawal, M. Saidani, L. Guillaud, M. Terenzio. Materials Science and Engineering: C 131, 112502 (2021). Featured in Materials Today.
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
Scientific Talks
- Hydrotropic regulation of neuronal proteins aggregation in synaptic organization and neurodegeneration – by Laurent Guillaud – October 11 2021 at the ADR2021 workshop - https://groups.oist.jp/adr/program
- Essential Role of Dyne Road Block1 in the survival of adult sensory neurons - 7th East Asia Joint Symposium - https://www.ims.u-tokyo.ac.jp/imsut/en/EAJS2021.html, 17 October 2021
- Investigating axonal molecular dynamics in regeneration and neurodegenerative diseases. Max Planck Institute for Polymer Research in Germany, 01 March 2022
Poster Presentations:
- Identifying interactors of DYNLRB1 in sensory neurons via proximity biotinylation - 8-9 September 2021 - https://dynein2021.org/
5. Intellectual Property Rights and Other Specific Achievements
Provisional patent application filing. Title: “Serotonin functionalized and melanin doped, Poly-hydroxyl butyrate Nano fibrous scaffold as a regenerative modality for neural tissue engineering and design of new hybrid fabrication system for the development of 3D scaffold”.
6. Meetings and Events
We organized the Axonal Degeneration and Regeneration Workshop at OIST: https://groups.oist.jp/adr
7. Other
7.1 Teaching
- Title: Neuronal Molecular Signaling
- Curriculum: Course A361 by Prof. Marco Terenzio.
- Date: Term 3 2021
- Venue: OIST campus.
- Lecturer: Marco Terenzio



