FY2021 Annual Report
Back row, left to right: Crystal Clitheroe, Soma Barawi, Vera Emelianenko, Kazuko Toyoda, Simon Hellemans, Nobuaki Mizumoto, Naoyuki Kuwahata.
Front row, left to right: Tereza Berankova, Menglin Wang, Anna Prokhorova, Esra Kaymak, Cedric Aumont, Ales Bucek, Tom Bourguignon.
The cute little boy in front: Lauro Bourguignon.
Missing: Yukihiro Kinjo, Kensei Kikuchi, Tracy Audicio, Jigyasa Arora.
Abstract
In FY2021, we continued our analysis of 50 termite genomes that we began sequencing in FY2020. These data are currently being processed and will be published in the next few years. For termites and cockroaches, we have sequenced approximately 1000 samples and will publish the data during the next few years. We also published nine peer-reviewed papers, three of which were published in Nature Index.
1. Staff
- Dr. Thomas Bourguignon, Assistant Professor
- Dr. Ales Bucek, Postdoctoral Scholar
- Dr. Yukihiro Kinjo, Postdoctoral Scholar
- Dr. Anna Prokhorova, Postdoctoral Scholar
- Dr. Simon Hellemans, JSPS Fellow/Postdoctoral Scholar
- Dr. Nobuaki Mizumoto, JSPS Fellow
- Crystal-Leigh Clitheroe, Research Unit Technician
- Esra Kaymak, Research Unit Technician
- Jigyasa Arora, Ph.D. Student
- Menglin Wang, Ph.D. Student/JRF
- Tracy Audicio, Ph.D. Student
- Kensei Kikuchi, Ph.D. Student
- Kazuko Toyoda, Research Unit Administrator
2. Collaborations
2.1 Historical biogeography of termites
- Type of collaboration: Joint research
- Researchers:
- Professor Yves Roisin, University of Brussels
- Professor Nathan Lo, University of Sydney
- Professor Rudolf A. Scheffrahn, University of Florida
- Professor Eliana Cancello, University of Sao Paulo
- Professor Xiaodong Yang, Xishuangbanna Tropical Botanical Garden
- Professor Brian Fisher, California Academy of Sciences
- Associate Professor Jan Sobotnik, Czech University of Life Sciences
- Associate Professor Theodore A. Evans, University of Western Australia
- Associate Professor David Sillam-Dusses, University of Paris 13
2.2 Functional evolution of termite gut microbes
- Type of collaboration: Joint research
- Researchers:
- Professor Andreas Brune, Max Planck Institute for Terrestrial Microbiology
- Professor Nathan Lo, University of Sydney
- Professor Gaku Tokuda, University of the Ryukyus
- Associate Professor Jan Sobotnik, Czech University of Life Sciences
2.3 Functional evolution of termite genomes
- Type of collaboration: Joint research
- Researchers:
- Associate Professor Dino McMahon, Free University of Berlin
- Associate Professor Jan Sobotnik, Czech University of Life Sciences
- Dr. Mark Harrison, University of Munster
3. Activities and Findings
3.1 Functional evolution of microorganisms in the termite gut
We used the gut metagenome of 221 termite samples to investigate the functional evolution of their gut microbiota. The termite species selected are representative of the phylogenetic and ecological diversity of termites, allowing us to investigate the functional evolution of termite gut microbiota thoroughly. During FY2021, we wrote one paper that was published in early FY2022. In this paper, we showed that (1) the gut prokaryotic genes involved in major nutritional functions and present in all termites have been acquired by a common ancestor, (2) the composition and function of termite gut microbial communities correlate with their phylogeny, and (3) the acquisition of a diet based on soil was not accompanied by the acquisition of new genes, but by a change in the stoichiometry of genes involved in important nutritional functions. Our results shed light on the taxonomic and functional evolution of the termite gut microbiota since they first appeared approximately 150 million years ago (Figure 1).
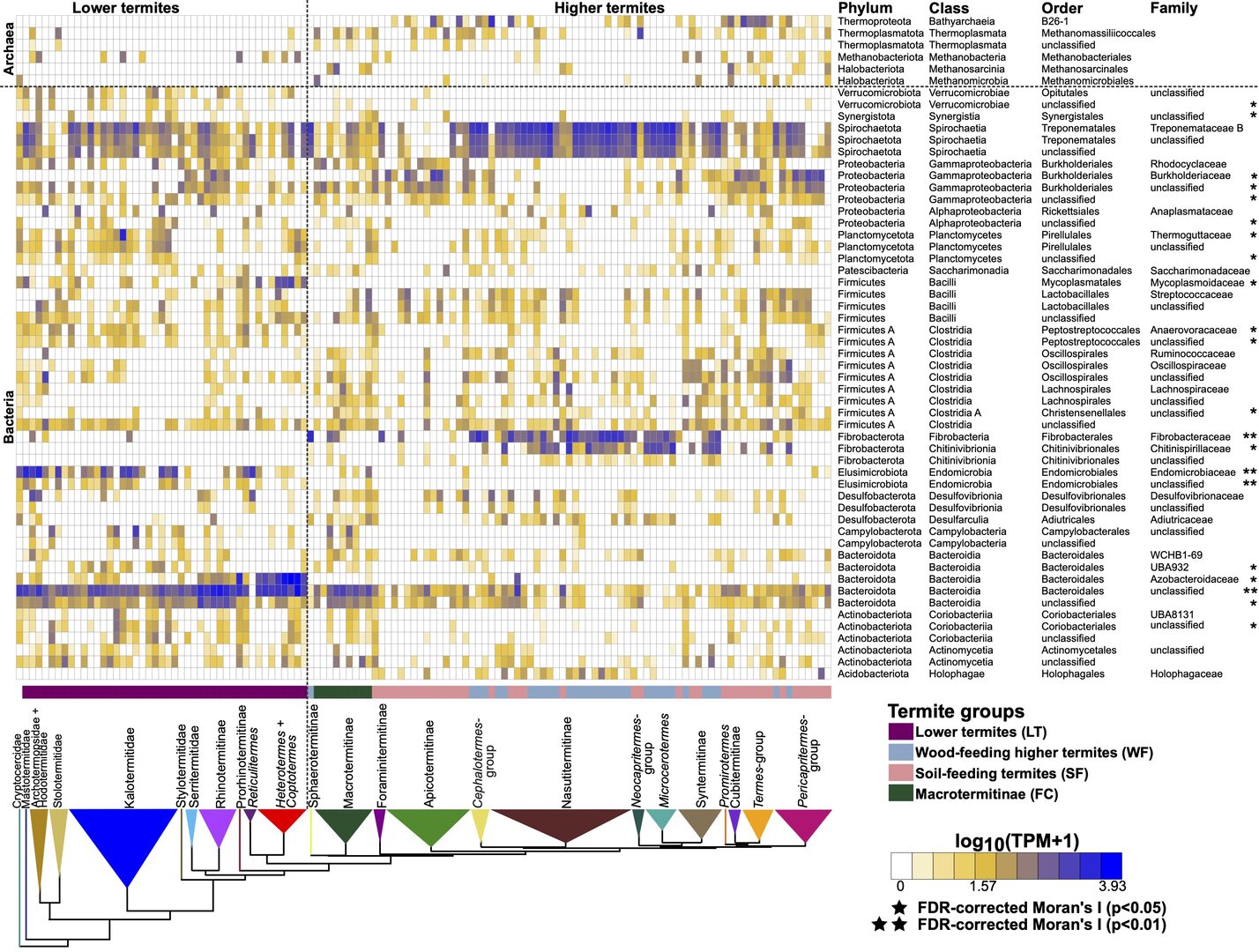
Figure 1: Relative abundance of the top 50 bacterial lineages and major archaeal orders found in termite gut metagenomes. The color scale represents the logarithm of transcripts per million (TPM). The tree is a simplified time-calibrated phylogenetic tree reconstructed using host termite mitochondrial genome sequences. Prokaryotic taxa that showed significant phylogenetic autocorrelation with host lineages at a false discovery rate (FDR) of 5% were assigned an asterisk (*p < 0.05; **p < 0.01). This figure was reproduced from Arora et al. 2022.
3.2 Evolution of genome size in the cockroach endosymbiont Blattabacterium
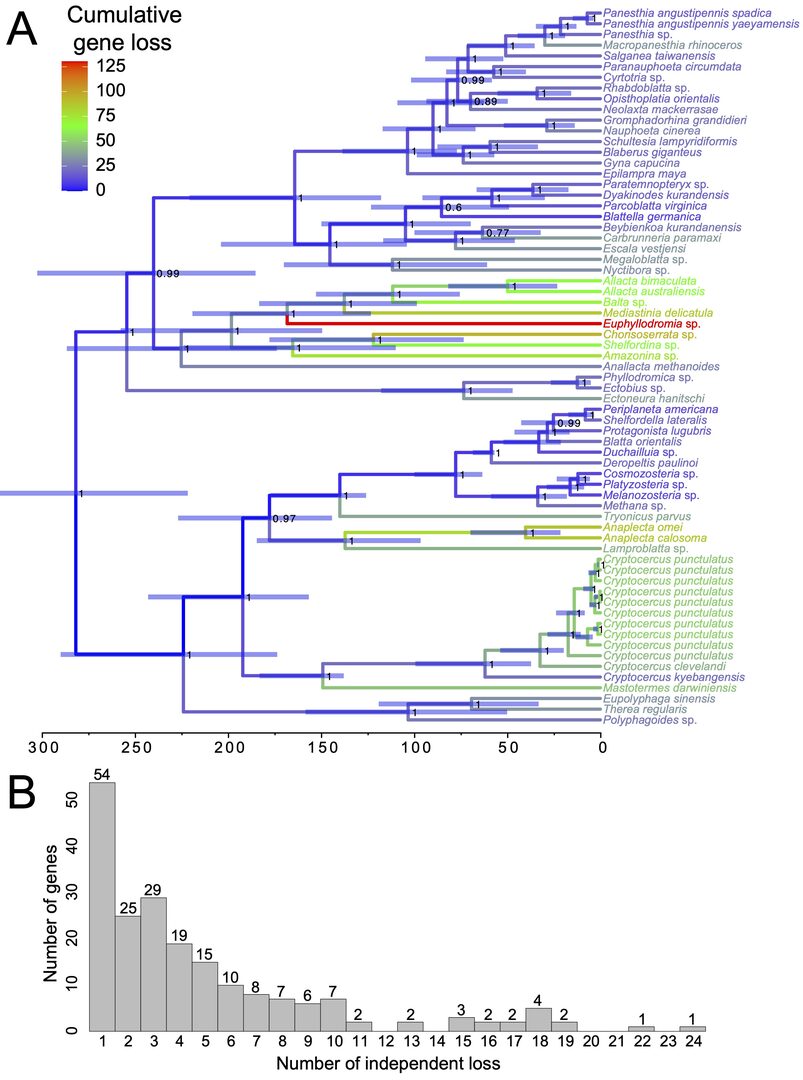
Figure 2: Evolution of genome reduction by gene loss in Blattabacterium. (A) Time-calibrated phylogenetic tree showing the evolution of genome reduction due to gene loss in Blattabacterium. This phylogenetic tree was reconstructed in BEAST using a set of 31 marker protein-coding genes. Branch colors represent cumulative gene loss. Node labels represent posterior distribution. Node bars indicate the 95% highest posterior density. (B) Histogram representing the frequency of independent gene losses estimated from 200 protein-coding genes lost in at least one lineage. The number above each bar indicates the frequency of independent gene loss. This figure was reproduced from Kinjo et al. (2021).
Most cockroaches live in symbiosis with Blattabacterium, a bacterial endosymbiont that provides nutrients to its host. Blattabacterium have been co-evolving with their cockroach host and vertically transmitted from mother to offspring for over 200 million years. The genome of Blattabacterium is five to ten times smaller than that of a typical free-living bacterium, and genes continue to be lost over a timescale of millions of years. We analyzed the genomes of 67 Blattabacterium species with the aim of determining the process of gene loss. We found that many genes are lost in parallel, and in the most extreme case, one gene is lost independently in 24 lineages (Figure 2). We identified three factors responsible for gene loss: (1) mutation rate, (2) relaxed selection on genes involved in vitamin and amino acid metabolism, and (3) domino effect, by which the loss of one gene leads to the loss of additional genes, due to epistatic interactions among genes. The results of this study was published during FY2021.
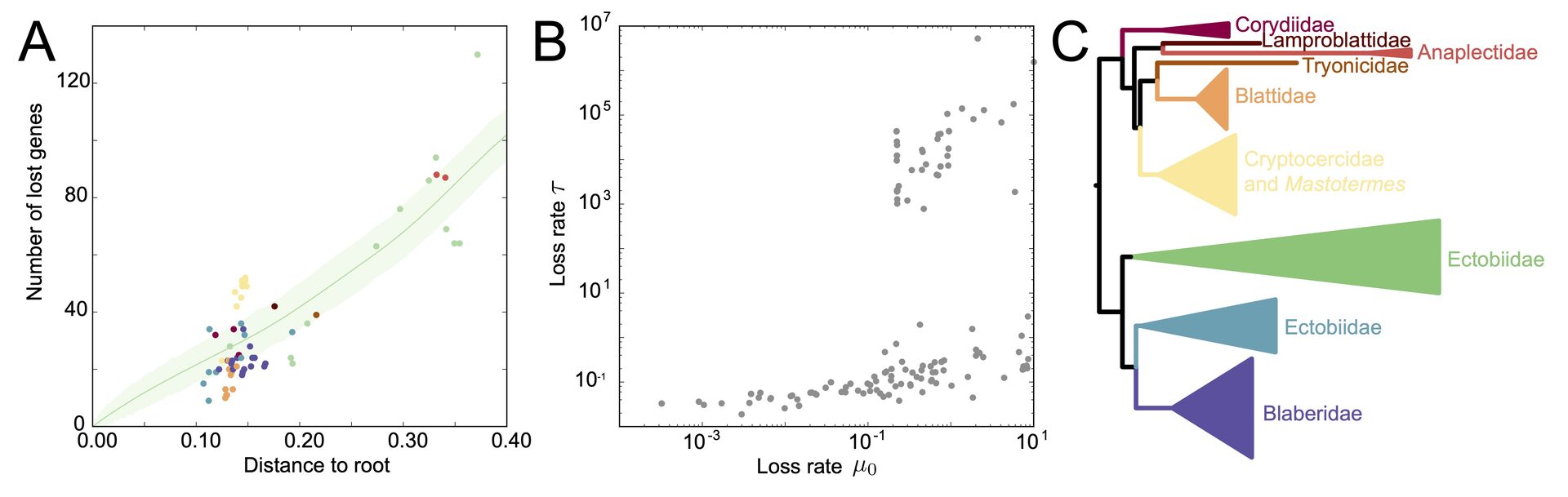
Figure 3: Exponential model examining gene loss as a function of substitution accumulation. (A) Exponential model of gene loss and the number of gene losses observed in Blattabacterium genomes. (B) Plot showing the two gene-specific loss parameters of the exponential model: the initial gene loss rate m0 and the exponential time scale s. (C) Phylogenetic tree of Blattabacterium. The colors of the branches correspond to the colors of the symbols in (A) (Reproduced from Kinjo et al. 2021).
3.3 Evolution of the termite genome
We started sequencing approximately 50 termite genomes during FY2019 with the goal of investigating how termite genomes have evolved over the past 150 million years. For each target genome, we generated long reads with the promethION platform and short reads with the Illumina platform for polishing. We also generated linked reads with Omni-C technology on the Illumina platform for scaffolding. The sequencing was completed during FY2021, and genome assembly was performed during FY2022 and FY2023. We plan to perform comparative genomics analyses during the next fiscal years.
4. Publications
4.1 Journals
- Beránková T, Buček A, Bourguignon T, Arias JR, Akama PD, Sillam-Dussès D, et al. The ultrastructure of the intramandibular gland in soldiers of the termite Machadotermes rigidus (Blattodea: Termitidae: Apicotermitinae). Arthropod Struct Dev 2022;67:101136. https://doi.org/10.1016/j.asd.2021.101136
- Yashiro, T., Tea, Y.-K., Van Der Wal, C., Nozaki, T., Mizumoto, N., Hellemans, S., Matsuura, K., Lo, N. 2021. Enhanced heterozygosity from male meiotic chromosome chains is superseded by hybrid female asexuality in termites. Proceedings of the National Academy of Sciences U.S.A. 118(51): e2009533118. https://doi.org/10.1073/pnas.2009533118
- Mizumoto N. & Bourguignon T. The evolution of body size in termites. (2021) Proceedings of the Royal Society B, 288: 20211458, https://doi.org/10.1098/rspb.2021.1458
- Mizumoto N., Lee S.B., Valentini G., Chouvenc T. & Pratt S.C. (2021) Coordination of movement via complementary interactions of leaders and followers in termite mating pairs. Proceedings of the Royal Society B, 288:20210998, https://doi.org/10.1098/rspb.2021.0998
- Che Y., Deng W., Li W., Zhang J., Kinjo Y., Tokuda G., Bourguignon T., Lo N. & Wang Z. (2022). Vicariance and dispersal events inferred from mitochondrial genomes and nuclear genes (18S, 28S) shaped global Cryptocercus distributions. Molecular Phylogenetic and Evolution 166, 107318. https://doi.org/10.1016/j.ympev.2021.107318
- Sillam-Dussès D., Hradecky J., Stiblik P., Ferreira da Cunha H., Carrijo T.F., Lacey M.J., Bourguignon T. & Šobotník J. (2021) The trail-following pheromone of the termite Serritermes serrifer. Chemoecology, 31, 11-17. https://doi.org/10.1007/s00049-020-00324-2
- Kinjo Y.*, Lo N.* (*equal first authors), Villa-Martin P., Tokuda G., Pigolotti S. & Bourguignon T. (2021) Enhanced mutation rate, relaxed selection, and the ‘domino effect’ are associated with gene loss in Blattabacterium, a cockroach endosymbiont. Molecular Biology and Evolution, 38, 3820-3831.https://doi.org/10.1093/molbev/msab159
- Beasley-Hall P.G., Rose H.A., Walker J., Kinjo Y., Bourguignon T. & Lo N. (2021) Digging deep: a revised phylogeny of Australian burrowing cockroaches (Blaberidae: Panesthiinae, Geoscapheinae) confirms extensive non-monophyly and provides insights into biogeography and evolution of burrowing. Systematic Entomology, 46, 767-783. https://doi.org/10.1111/syen.12487
- Romero Arias J., Boom A., Wang M., Clitheroe C., Šobotník J., Stiblik P., Bourguignon T. & Roisin Y. (2021) Molecular phylogeny and historical biogeography of Apicotermitinae (Blattodea: Termitidae). Systematic Entomology, 46, 741-756. https://doi.org/10.1111/syen.12486
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Kinjo Y. An introduction to Blattabacterium, the endosymbiont of cockroaches. 81th Annual meeting of the Entomological Society of Japan, Japan, September 2021
- Kinjo Y., Thomas Bourguignon. Three mechanisms driving reductive genome evolution of Blattabacterium, the endosymbiont of cockroaches. Annual meeting of Society of Genome Microbiology Japan, Japan, March 2022.
- Kikuchi K., Kimura A., Mitarai E., Miyazato M., & Mizumoto N. Nest is an indicator of inherent worker movements in termites. ISMMA 2021, Online, January 2022, (Poster)
- Lynch C., Starkey M., Pavlic P., Montgomery D., & Mizumoto N. A Study on Optimal Sampling in Multiple Social Insect Colonies with a Model-Based Approach. ABA 2021 Virtual Meeting, August 2021, (Poster)
- Mizumoto N., Bardunias P. M. & Pratt C. S. Parameter Tuning Facilitates the Evolution of Diverse Tunneling Patterns in Termites. DARS-SWARM 2021, Online, June 2021 (oral)
- Mizumoto N. Same-sex pairing is maintained by acting the other sex in termites. Ethological Society Japan, September 2021, Online
- Mizumoto N., Evolutionary perspectives of collective animal behavior, The Annual Meeting of the Japan Ethological Society (Tokyo) Sep. 2021 (Oral) (Invited)
- Mizumoto N., Comparative approach to understanding behavioral rules of termites, nest building and movement coordination, Virginia Tech University, Department of Entomology, Feb. 2022 (Invited)
- Mizumoto N., Evolutionary perspectives of termite collective behavior. Mississippi State University, Department of Biology, online, Feb. 2022
- Mizumoto N., Evolutionary perspectives of termite collective behavior. University of South Florida, Department of Integrative Biology, online, Jan. 2022
- Mizumoto N., Comparative analysis of behavioral rules in termites, nest building and pair movement coordination., ICMMA 2021, online, invited speaker, Jan. 2022
- Mizumoto N., Behavioural mechanism and its evolutionary history of termite tunnel excavation, EU-IUSSI online symposium series, Oct. 2021
- Mizumoto N., Same-sex pairing is maintained by acting the other sex in termites. University of St. Andrews, online, Sep. 2021
- Bourguignon T., Genome evolution of the cockroach endosymbiont, Blattabacterium cuenoti. Seminar in Ecology and Evolution of the Institute of Biology of the Freie Universitat Berlin, Germany. 26 April 2021.
- Wang M. Neoisoptera repetitively colonised Madagascar after the Middle Miocene climatic optimum. 69th Annual Meeting of Ecological Society of Japan (ESJ69). Mar. 2022. (Oral)
- Buček A. Convergent evolution of defensive mandibular adaptations in termites. 69th Annual Meeting of Ecological Society of Japan (ESJ69). Mar. 2022. (Oral)
- Prokhorova A. Long-term bioelectrochemical treatment of livestock wastewater in pilot-scale using electrotrophic bacteria. 34th Annual Meeting of the Japanese Society of Microbial Ecology (JSME). Nov. 2021. (Oral)
- Prokhorova A. Microenvironmental heterogeneity in gut of Nasutitermes drives differential ecological specificity and function of bacterial symbiont communities. 69th Annual Meeting of Ecological Society of Japan (ESJ69). Mar. 2022. (Oral)
- Mizumoto N., Bourguignon T., Bailey N., Flexible sexual role maintains same-sex pairing in termites, March 2022, Online (Poster)
- Bourguignon T. The functional evolution of termite gut microbiota. JSME conference (34th Annual Meeting of the Japanese Society of Microbial Ecology (JSME). Nov. 2021. (Oral, Invited))
5. Intellectual Property Rights and Other Specific Achievements
- Grant-in-Aid for Scientific Research (B) (Kakenhi), Japan Society for the Promotion of Science, "Elucidation of the mutation rate as driver of insect endosymbiont genome evolution", Lead PI: Bourguignon, T., Period: April 2021–March 2023
タイトル英訳:Elucidating the dynamics and driving factors of mutation rate evolution in endosymbiotic bacteria - Grant-in-Aid for Early-Career Scientists, Japan Society for the Promotion of Science, "細胞内共生細菌における突然変異率の進化動態とその駆動要因の解明", Lead PI: Kinjo, Y., Period: April 2021–March 2023
タイトル英訳:Elucidating the dynamics and driving factors of mutation rate evolution in endosymbiotic bacteria - The Motoo Kimura Trust Foundation for the Promotion of Evolutionary Biology, for Japan Eco-Evo English Seminar
- 37th Inoue Research Award for Young Scientists, Grant-in-Aid for JSPS Fellows, Japan Society for the Promotion of Science, "Evolutionary process of termite construction revealed by comparative and constructive approaches", Lead PI: Mizumoto, N., Amount: 11.7M Yen, Period: April 2020–March 2023
- Research Fellowship for Young Scientists (DC2), Japan Society for the Promotion of Science, "シロアリにおけるゲノム構造と化学受容体遺伝子族の進化", Lead PI: Tracy Audisio, Period: April 2021–March 2023
- Grant-in-Aid for Young Scientists, Japan Society for the Promotion of Science, "シロアリにおけるゲノム構造と化学受容体遺伝子族の進化", Lead PI: Tracy Audisio, Period: April 2021–March 2023
6. Meetings and Events
6.1 Japan Eco-Evo English Seminar #1-#7
https://sites.google.com/view/jee-english-seminar
6.2 Ethological Society Japan, Round table,
アート X 動物行動学:科学の外にある表現方法の可能性, Mizumoto N., Kikuchi K.




