FY2016 Annual Report
Cellular and Molecular Synaptic Function Unit
Distinguished Professor (Fellow) Tomoyuki Takahashi

Abstract
In the presynaptic terminal, neurotransmitters are filled in synaptic vesicles and released by vesicle exocytosis. After exocytosis, vesicle membranes are retrieved by endocytosis and refilled with neurotransmitter actively taken up from presynaptic cytosol to be reused for the next round of neurotransmission. At glutamatergic synapses, glutamate is concentrated in synaptic vesicles at ~ 100 mM, that is ~10 times higher than its cytosolic concentration. Therefore, it is thought that glutamate can passively leak out of vesicles. In fact, in isolated vesicles, isotope-labeled glutamate leaks out of vesicles. However, it is controversial whether glutamate leaks out of vesicles in presynaptic terminals. Using the giant synapse calyx of Held visualized in rodent brainstem slices, we investigate whether glutamate leaks out of vesicles, and if it does, to what extent. To this aim, we washed out presynaptic cytosolic glutamate using whole cell recording pipette containing no glutamate and monitored the amplitude of spontaneous miniature, i.e. single vesicular, EPSCs (mEPSCs) and multi-vesicular EPSCs evoked only infrequently (1/10 min) by presynaptic action potentials, in simultaneous presynaptic and postsynaptic patch-clamp recording. Our results indicated a steep decline of evoked EPSC amplitudes, but much less reduction of mEPSC amplitude over 30 min after washout cytosolic glutamate. We estimated the number of vesicles, which underwent recycling after evoked and spontaneous exocytosis during 30 min period. From these results, we conclude that leakage of glutamate from vesicles does occur in the absence of cytosolic glutamate, but plays a minor role in the rundown of evoked EPSC amplitude, which is mainly caused by accumulation of empty vesicles in presynaptic terminals retrieved after spontaneous and evoked glutamate release.
Chihiro Takami, Kohgaku Eguchi, Tetsuya Hori, Tomoyuki Takahashi (2017) J Physiol 595, 1263-1271.
1. Staff
- Dr. Hiroshi Takagi, Group Leader
- Dr. Kohgaku Eguchi, Staff Scientist (until January, 2017)
- Dr. Laurent Guillaud, Staff Scientist
- Dr. Zacharie Taoufiq, Staff Scientist
- Dr. Alemeh Zamani, Postdoctoral Scholer (from October, 2016)
- Dr. Dimitar Dimitrov, Specialist, Technician
- Ms. Anna Garanzini, Technician
- Mr. Francois Beauchain, Technician (from April, 2016)
- Dr. Han-Ying Wang, Visiting Researcher (from May, 2016)
- Ms. Lashmi Piriya Ananda Babu, OIST Ph.D. Student
- Ms. Larisa Sheloukhova, Lab Rotation Student (from January to April, 2016)
- Ms. Aliya Mari Adefuin, Rotation Student (from May to August, 2016)
- Ms. Mai Ahmed, Lab Rotation Student (from July to December, 2016)
- Ms. Swathy Babu, Lab Rotation Student (from January, 2016)
- Ms. Manana Kutsia, Lab Rotation Student (from January, 2016)
- Mr. Ke Wang, Lab Rotation Student (from January, 2016)
- Ms. Sayori Gordon, Research Unit Administrater
2. Collaborations
2.1 Research Theme: Regulatory mechanisms for transmitter
release
- Type of partnership: Joint research
- Researchers:
- Principal researcher: Associate professor Tetsuya Hori, Doshisha University
- Dr. Manami Yamashita, Doshisha University
- Associate professor Naoto Saitoh, Doshisha University
- Name of partner organization: Doshisha University, Faculty of Life and Medical Sciences
2.2 Research Theme: Effect of photo-switchable microtubules
disassembler PST on α-synclein-induced inhibition of vesicle
endocytosis
- Type of partnership: Scientific Collaboration
- Researchers:
- Principal researcher: Professor Dirk Trauner, Ludwig-Maximillians-University
- Dr. Oliver Thorn-Seshold, Ludwig-Maximillians-University
- Name of partner organization: Department of Chemistry and Pharmacy and Centre for Integrated Protein Science, Ludwig-Maximillians-University
3. Activities and Findings
3.1 Impact of vesicular glutamate leakage on synaptic transmission at the calyx of Held. (Takami et al, 2017, J Physiol 595, 1263-1271)
In simultaneous pre- and postsynaptic recording, in the absence of glutamate in presynaptic pipette, cytosolic glutamate is washed out by pipette solution having a much larger volume than the terminasl. As glutamate washout proceeds, both evoked and spontanaeous miniature (m) EPSCs undergo rundowns in amplitude (Fig 1A, B). The rate of rundown, however, is much less for mEPSCs. Such a dissociation has been observed previously (Zhou et al, 2000; Ishikawa et al, 2002), but there is no convincing explanation. Since the limited reduction of mEPSCs has been attributed to low signal to noise ratio of mEPSC recording, we analyzed mEPSC amplitude histogram (open coulms, Fig1D) in comparison to noise histograms (filled colums) showing that both histograms are well separated.
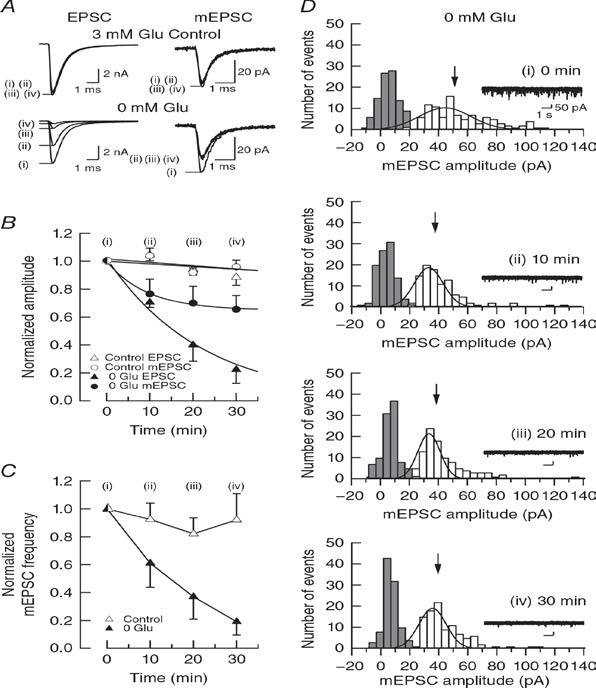
Figure 1
Rundown of EPSCs occurs without glutamate washout when vesiclular acidification is inhibited using the vacuolar ATPase inhibitor bafilomycin A1.Bafilomycin block vesicle acidification, thereby blocking refilling newly endocytosed vesicels with glutamate. In hippocampal glutamatergic synapses in culture, bafilomycin causes rundown of evoked EPSCs, but it is controversial whether it causes rundown of mEPSCs due to passive glutamate leakage out of vesicles. We re-examine this issue and found that bafilomycin attenuates mEPSCs (Fig 2) to a similar extent as in experiments of glutamate washout (Fig 1).
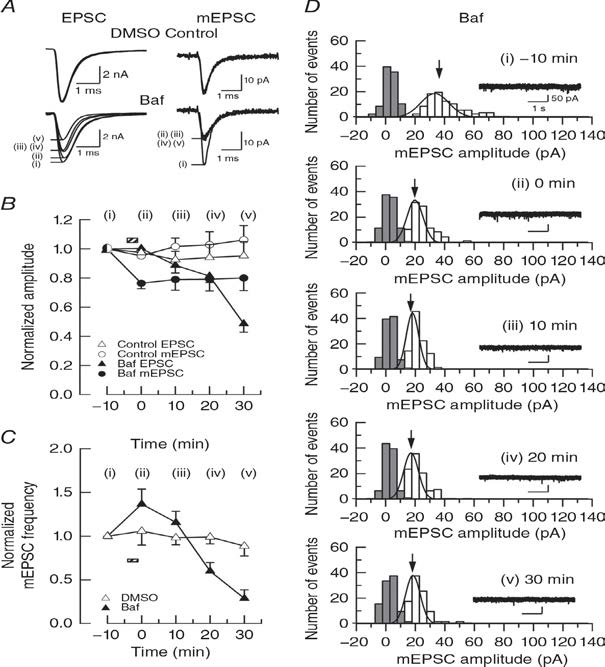
Figure 2
Previous report (Zhou et al, 2000) attributed the smaller amplitude reduction of mEPSCs compared with evoked EPSCs during bafilomycin application to a low detectability of mEPSCs because their amplitude is close to background noise level. To evaluate this explanation, we reduced mEPSC amplitude by blocking postsynaptic AMPA receptors using CNQX (Fig 3). Titration of CNQX from low to high concentrations, attenuated mEPSC amplitude in a concentration dependent manner. In parallel, evoked EPSC amplitude was attenuated to a similar extent (Fig 3B). CNQX at 1.5 mM attenuated mEPSCs to 19% (Fig 3B), but these events are still clearly detected in separation from noise histogram (Fig 3E).
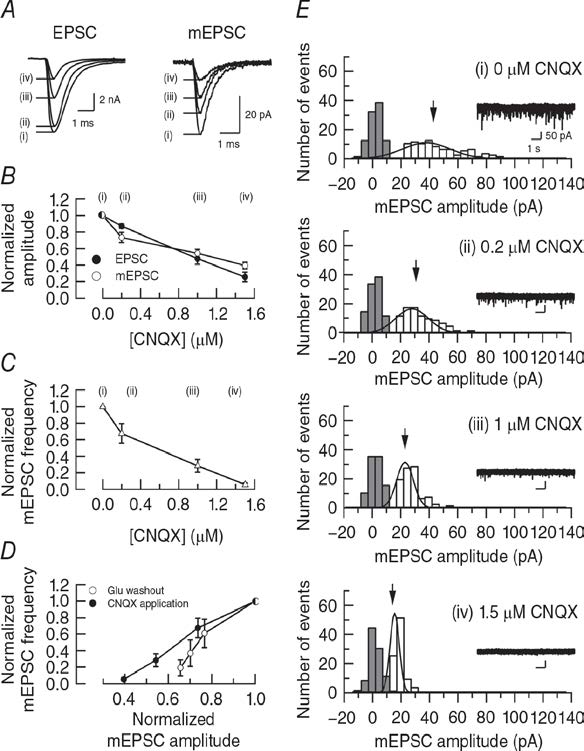
Figure 3
Another interpretation for the dissociation in the magnitude of rundown between mEPSCs and EPSCS is a possible reduction of release probability after glutamate washout from cytosol as suggested in cultured hippocampal autapses (Herman et al, 2014). We tested this possibility using presynaptic membrane capacitance measurements. We evoked exo-endocytosis of synaptic vesicles by a square pulse- elicited Ca2+ currents in the presence of glutamate and in its absence (Fig 4). Our results indicate no difference in exocytic magnitude. Thus, release probability is unaffected by glutamate washout.
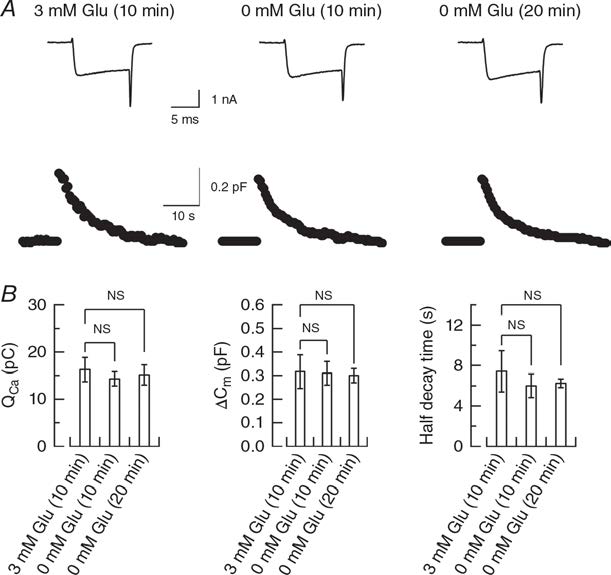
Figure 4
These results altogether indicate that glutamate can leak out of vesicles when its uptake is blocked. The greater reduction of evoked EPSCs is likely due to recycling replenishment of empty vesicles that occurs after spontaneous and evoked exocytosis. Since the mEPSC amplitude represents events produced by filled vesicles, their reduction reflects vesicular glutamate leakage more faithfully. Bafilomycin is conventionally utilized for counting the number of recycling vesicles in such a way to divide time integral of evoked EPSC amplitude by mEPSC amplitude, assuming no leakage of vesicular neurotransmitter (eg Ikeda & Bekkers, 2009). Such calculation needs corrections of glutamate leakage and more importantly the number of recycling vesicles after spontaneous exo-endocytosis from mEPSC frequency during the recording period.
3.2 Presynaptic morphology and vesicular composition determine vesicle dynamics in mouse central synapses. (Guillaud et al, 2017, eLIFE, e24845)
We characterized synaptic vesicle dynamics in the calyceal terminals in culture. In resting terminals labeled vesicles moved with irregular speeds and various trajectories, indicating that SV movements were more heterogeneous than previously thought. After fixation of the cells with aldehyde, SV mobility dropped down to 1/8, providing a good S/N ratio for SV imaging. Using IMARIS software, we analyzed SV movements quantitatively. SVs traveling a longer distance generally had a higher maximal speed. The SV mobility in calyceal terminal swelling was twice higher than that in hippocampal presynaptic boutons when compared for similar size structures. Percentage of long SV trafficking (trajectory length > 4 µm) decreased from 30% to 10% as presynaptic terminals undergo structural development from stage 1/2 (immature) to 3/4 (mature). When Venus-VGLUT1 and Venus-VGLUT2 were over-expressed into separate CN neurons, Venus-VGLUT1 SVs showed higher mobility than Venus-VGLUT2 SVs. Furthermore, track length and max speed were higher and SVs with active directional modality were more frequently observed in Venus-VGLUT1 SVs. These results suggest that the molecular signature of SVs can affect their mobility. Disrupting microtubules assembly using nocodazole preferentially reduced long/fast traffics, suggesting that microtubules are involved in inter-synaptic SV trafficking. When vesicle exocytosis was triggered by bath-application of 65 mM [KCl] solution, many SVs underwent exocytosis, long/fast SV movements were significantly reduced, and remaining SVs became immobile. Hypertonic sucrose solution had no effect on SV mobility, but reduced directional modality of SVs. Electrical stimulation at 1 Hz had no appreciable effect on vesicle mobility outside or within active zone.
3.3 Wild-type monomeric a-synuclein can impair vesicle endocytosis and synaptic fidelity via tubulin polymerization at the calyx of Held. (Eguchi et al, 2017, J Neurosci 37:6043-6052)
a-Synuclein is an endogenous protein normally attached to vesicular or plasma membranes in the nerve terminal. Excess a-synuclein is known to accompany a-synucleinopathy including Parkinson’s disease. We have loaded a-synuclein into calyx terminals in acute slices and found that it slows endocytosis with no immediate effect on exocytosis or ICa. This inhibitory effect of a-synuclein on vesicle endocytosis was rescued by co-loading the microtubules depolymerizing drug nocodazole in nerve terminals. Furthermore, a photo-switchable microtubules depolymerizer photostatin co-loaded with a-synuclein rescued the latter effect under 390 nm illumination, but its rescuing effect was reversed at 510 nm illumination. a-synuclein has little effect on basal neurotransmission, but slows recovery of EPSCs from short-term synaptic depression, thereby impairing fidelity of high frequency neurotransmission. Nocodazole rescued all these defects caused by a-synuclein. These results suggest that microtubules over-assembly underlie the loss of function caused by a-synuclein loading.
3.4 Presynaptic molecular mechanism underlying ischemic long-term potentiation (Takagi et al, in preparation).
When brains are in ischemic state, neurons start to degenerate because of glutamate efflux from cells. Hippocampal CA1 region is known to be one of the most sensitive regions to ischemic insult. In fact, glutamatergic field EPSPs recorded from CA1 undergo long-term potentiation upon application of oxygen/glucose deficient (OGD) solution in hippocampal slices. This ischemic LTP (iLTP) is induced presynaptically because it accompanies a decrease in the PPR (see above). After experiments using pharmacological tools, we found that the presynaptic mechanism underlying iLTP largely overlap with that operating for exo-endocytic balancing at the calyx of Held (Eguchi et al, 2012 Neuron) as well at hippocampal synapses in culture (Micheva et al, 2003 Nat Neurosci). Namely, both the iLTP and exo-endocytic balancing mechanisms are mediated by a signaling molecule cascade including NMDA receptor, NO, protein kinase G, Rho kinase, and PIP2. On top of it our results suggest involvement of astrocyte in the upstream of NO.
3.5 Proteomics of presynaptic proteins (Taoufiq et al, manuscript in preparation).
We developed ultra-high resolution mas-spectrum analysis and detected >5,000 proteins in synaptosomal fraction from P20 rat brain tissue. We further dissected synaptosomes into free vesicle, cytosol, membrane and mitochondrial fractions and quantitated copy numbers of individual proteins in each fractions. After confirming that typical vesicle proteins or active zone (AZ) proteins are predominantly in free vesicle or membrane fractions including AZ, we found many other proteins residing in both free vesicle and AZ membrane fraction in different quantities. We have also found new presynaptic proteins and unexpected expression patterns of known proteins.
4. Publications
4.1 Journals (OIST researchers are underlined)
1. Chihiro Takami, Kohgaku Eguchi, Tetsuya Hori, Tomoyuki Takahashi (2017) Impact of vesicular
glutamate leakage on synaptic transmission at the calyx of Held. JPhysiol 595, 1263-1271.
2. Dimitar Dimitrov, Hiroshi Takagi, Laurent Guillaud, Naoto Saitoh, Kohgaku Eguchi,
Tomoyuki Takahashi (2016) Reconstitution of giant mammalian synapses in culture for molecular
functional and imaging studies. J Neurosci 36, 3600-3610.
3. Yusuke Mori, Kohgaku Eguchi, Kiyonori Yoshii, Yoshitaka Ohtubo, (2016). "Selective expression
of muscarinic acetylcholine receptor subtype M3 by mouse type III taste bud cells." Pflugers
Archiv – European Journal of Physiology, Doi:10.1007/s00424-016-1879-5.
4.2 Books and other one-time publications
Nothing to report
4.3 Oral Presentations
Nothing to report
4.4 Poster Presentation
Eguchi, K., Taoufiq, Z., Takahashi, T., Acute increase of α-synuclein in presynaptic terminals impairs synaptic fidelity via excessive microtubule assembly at glutamatergic synapses. Federation of European Neuroscience Societies Forum 2016. Copenhagen, Denmark. July 2-6,2016
Eguchi, K., Taoufiq, Z., Takahashi, T., Acute increase of α-synuclein in presynaptic terminals impairs synaptic fidelity via excessive microtubule assembly at glutamatergic synapses. The OIST Computational Neuroscience Course 2016. OIST seaside house. July 13-30, 2016
Guillaud, L., Dimitrov, D., Takahashi, T., Presynaptic morphology and vesicular protein composition influence vesicle dynamics in central synapses. JSPS Core-to-Core symposium & OIST Joint Symposium. OIST, September 25-27, 2016
Eguchi, K., Taoufiq, Z., Takahashi, T., Pathological effects of wild-type and mutant alpha-synuclein on synaptic transmission at glutamatergic synapses. JSPS Core-to-Core symposium & OIST Joint Symposium. OIST, September 25-27, 2016
Taoufiq, Z., Ninov, M., Villar-Briones, A., Sasaki, T., Jahn, R., Takahashi, T., Ultradefinition & Automated Synapse Proteomics. JSPS Core-to-Core symposium & OIST Joint Symposium. OIST, September 25-27, 2016
Dimitrov, D., Eguchi, K., Guillaud, L., Takagi, H., Nakanishi, S., Takahashi, T., Distinct TrkC domains regulate structural and functional maturation of calyx-like giant synapses in dissociated cell culture. JSPS Core-to-Core symposium & OIST Joint Symposium. OIST, September 25-27, 2016
Ananda Babu, L. P., Guillaud, L., Eguchi, K., Takahashi, T., Presynaptic microtubules are involved in the maintenance of synaptic transmission. JSPS Core-to-Core symposium & OIST Joint Symposium. OIST, September 25-27, 2016
Guillaud, L., Dimitrov, D., Takahashi, T., Presynaptic Morphology and Vesicular Compostion Influence Vesicle Dynamics in Central Synapses. Annual meeting of the American Society for Cell Biology. San Francisco, U.S.A. December 3-7, 2016
5. Intellectual Property Rights and Other Specific Achievements
5.1 Grant
Kohgaku Eguchi: Grant-in-Aid for Young Scientists B, KAKENHI (2015-2017)
6. Meetings and Events
6.1 JSPS Core-to-Core Program & OIST Joint Symposium
Title: Nanoscopic Synaptic Functions
Co-organizer:
Prof. Tomoyuki Takahashi (OIST), Prof. Takeshi Sakaba (Doshisha University)
Date: September 25 – September 27, 2016
Venue: OIST Campus
Speakers (in alphabetical order):
- Haruhiko Bito (University of Tokyo)
- Nils Brose (Max Planck Institute)
- Robert Edwards (University California)
- Ian Forsythe (University of Leicester)
- Stefan Hallermann (Leipzig University)
- Volker Haucke (FMP Berlin)
- Reinhard Jahn (Max Planck Institute for Biophysical Chemistry)
- Peter Jonas (Institute of Science and Technology Austria)
- Ege Kavalali (UT Southwestern Medical Center)
- Shinya Kawaguchi (Doshisha University)
- Alain Marty (University Paris 5)
- Ko Matsui (Tohoku University)
- Hiroaki Misonou (Doshisha University)
- Sumiko Mochida (Tokyo Medical University)
- Tobias Moser (University Medical Center Göttingen)
- Ryuichi Shigemoto (Institute of Science and Technology Austria)
- Stephan Sigrist (Freie University of Berlin)
- Tomoyuki Takahashi (Okinawa Institute Science and Technology Graduate University)
- Shigeo Takamori (Doshisha University)
- Laurence Trussell (Vollum Institute Portland)
- Michisuke Yuzaki (Keio University)
6.2 Invited SeminarDate: May 9, 2016
- Venue: Research Institute of Environmental Medicine, Nagoya University
- Speaker: Professor Tomoyuki Takahashi
- Title: Regulatory mechanisms of synaptic vesicle recycling
- Date: September 27, 2016
- Venue: OIST Center Buildings
- Speaker: Professor Tomoyuki Takahashi
- Title: Physiological and Pathological Involvements of Microtubules in Presynaptic Functions
- Date: July 7, 2016
- Venue: The Institute of Science and Technology Austria
- Speaker: Kohgaku Eguchi
- Title: Acute increase in alpha-synuclein impairs the fidelity of synaptic transmission at calyx
of Held synapses
6.3 OIST Seminar
- Date: December 16, 2016
- Venue: OIST Campus, Lab 1
- Speaker: Dr. Yasunori Hayashi, RIKEN Brain Science Institute
- Title: Molecular Mechanisms of Hippocampal Synaptic Plasticity
- Date: February 14, 2017
- Venue: OIST Campus, Lab 1
- Speaker: Dr. Elizabeth Theriault, Research and Informatics at the Ontario Brain
Institute,Toronto, Ontario - Title: Developing a Learning Healthcare System: The Ontario Brain Institute
Model.
- Date: March 24, 2017
- Venue: OIST Campus, Lab 1
- Speaker: Dr. Takafumi Miki, Laboratoire de Physiologie Cérébrale, Université
Paris Descartes - Title: Estimating docking site number and releasing vesicles reveals two
sequential vesicular pool model at single glutamatergic synapses
7. Other
7.1: Teaching class ‘Introduction to super resolution microscopy (nanoscopy)
- Imaging beyond the diffraction limit’, part of the 'Emerging technologies in life sciences' course. For the first year PhD students organized by Pr. Ichiro Maruyama, OIST
- Date: July 29, 2016
- Lecturer: Laurent Gillaud



