Invited Speakers

Invited Speakers
The scope of the symposium is aligned with OIST’s mission “to conduct internationally outstanding education and research in science and technology, and to promote and sustain the advancement of science and technology in Japan and throughout the world”. An outstanding and eclectic lineup of internationally acclaimed researchers has been selected as invited speakers. To capture the multi- and interdisciplinarity of nanotechnology, leading figures in cluster beam deposition will share their state-of-the-art expertise on various aspects of the field with different perspectives, according to their individual backgrounds, which range from materials science and physics to chemistry and biology, both theoretical and experimental. Apart from transcending scientific boundaries, the proposed symposium aspires also to transcend geographic boundaries as the invited speakers come from all over the world.

Kinetics of structural transformations of metallic nanoparticles and nanoalloys
The experimentally observed nanoparticle structures are often the result of a competition between kinetic effects and equilibration. Here we consider different types of processes, ranging from temperature-dependent solid-solid transformations [1-5], to the transition from the liquid to the solid state at decreasing temperature [6], to growth processes in gas phase [7,8]. The systems considered here are pure Au nanoparticles [3,5], and several different nanoalloys (AgPt [4], AuCo, AgCu, AgNi [5,6], AgAu [8]). Kinetic effects are studied by classical molecular dynamics, while equilibrium properties are determined by global optimization searches, harmonic thermodynamics calculations and DFT calculations. We show how simulation results can help in interpreting complex processes in different experiments [3,4,7,8].
[1] E. Panizon, R. Ferrando, Phys. Rev. B 92, 205417 (2015)
[2] E. Panizon, R. Ferrando, Nanoscale 8, 15911 (2016)
[3] D.M. Foster, R. Ferrando, R.E. Palmer , Nature Communications 9, 1323 (2018)
[4] J. Pirart, A. Front, D. Rapetti, P. Andreazza, C. Andreazza, C. Mottet, R. Ferrando, Nature Communications, in press (2019).
[5] D. Nelli, R. Ferrando, submitted (2019)
[6] J.-P. Palomares-Baez, E. Panizon, R. Ferrando, Nano Letters, 17, 5394 (2017)
[7] D.M. Wells, G. Rossi. R. Ferrando, R.E. Palmer, Nanoscale, 7, 6498 (2015)
[8] T.-W. Liao, A. Yadav, K.-J. Hu, J. van der Tol, S. Cosentino, F. D'Acapito, R.E. Palmer, C. Lenardi, R. Ferrando, D. Grandjean, P. Lievens, Nanoscale 10, 6684 (2018)

Recent steps in improving yield and stability of magnetron cluster source and applications
Since its birth [1], magnetron based cluster beam deposition has demonstrated to be a unique tool for the fabrication of pristine nanoparticles of high interest for fundamental studies as well as for technological applications. Surprisingly the gas aggregation sources (GAS) have not penetrated yet the industrial sector although their use for several applications has been proposed.
In this talk I will discuss some of the issues of the GAS that may explain why they are not implemented in industrial processes as the limitation of the yield of nanoparticles and the short time stability. Possible solutions to overcome these limitations will be presented like the use of a Full Face Erosion magnetron and the injection of controlled doses of gas impurities [2,3]. I will show that stable and high fluxes of nanoparticles open the route to the fabrication of three-dimensional coral-like structures that present a high effective surface.
Finally I will present three applications where nanoparticles generated by GAS have been used for high performance scanning probes [4], nanomedicine and photocatalysis.
[1] H. Haberland et al., Z. Phys. D: At., Mol. Clusters 20, 413 (1991).
[2] "Gas Phase Synthesis of Nanoparticles”, Wiley-VCH Verlag GmbH, 2017.
[3] Y. Huttel et al., MRS Communications 8, 947 (2018).
[4] L. Martínez et al., Review of Scientific Instruments, 82, 023710 (2011).

Bimetallic catalysts produced by cluster beam deposition
Our ambition is to design catalysts through advanced atomic scale understanding. Combining analytical and in-situ/operando tools with computational methods is an exciting approach to identify next-generation (electro)catalysts for energy transition and biosensing applications. This requires the production of homogeneous systems as is possible with cluster beam deposition (CBD) technology. We investigated the relationship between structure and properties at atomic level of a series of novel catalysts with ultra-low loading of supported bimetallic clusters of uniform size and composition.1,2 The cluster catalysts were characterized using electron microscopy (SEM and STEM), X-ray photoelectron spectroscopy (XPS), X-ray absorption fine structure (XAFS), diffuse reflectance infrared Fourier transform (DRIFT) spectroscopies, temperature programmed desorption (TPD), gas-phase reactions, and electrochemical methods.
Several examples of bimetallic clusters applied as (electro)catalysts are discussed, highlighting the potential of CBD to design and optimize next generation supported cluster-based catalysts and heterogeneous devices.
A highly efficient methanol dehydrogenation catalysts based on PtNi bimetallic NCs with composition-tuned structures was developed and tested by TPD. Pt-skins formed at the surfaces of Pt0.7Ni0.3 clusters were found to catalyze the decomposition of methanol below 0 °C, with a significant reduction of the CO poisoning effect.3 A low temperature CO oxidation catalyst based on PdNi clusters directly deposited on alumina powder was produced. Several potential active sites responsible for the catalyst activity were identified by DRIFT under CO oxidation conditions.4 In a model electrocatalyst for the oxygen evolution reaction under alkaline conditions consisting of Ni0.5Fe0.5 clusters deposited on a conducting FTO electrode, we showed with XAFS and XPS that after reaction the cluster surface was enriched in NiOOH and depleted in Fe.5 A biosensor was produced based on PtNi clusters deposited on a conducting polyaniline polymer for the detection of dopamine, a major excitatory neurotransmitter of the human brain. Its high performance was attributed to the formation of mixed Pt metal and Ni-O(OH) phases at the surface of the alloyed PtNi cores of Pt0.7Ni0.3 under electrochemical conditions.6
We acknowledge support from the EU 7th Framework Programme (FP7/2007-2013, GA no. 607417 (Catsense), the KU Leuven Research Council (CELSA/18/032), and the Research Foundation - Flanders (FWO) (G.0B39.15).
[1] Liao, T.-W.; Yadav, A.; Hu, K.-J.; van der Tol, J.; Cosentino, S.; D'Acapito, F.; Palmer, R.E.; Lenardi, C.; Ferrando, R.; Grandjean, D.; Lievens, P. Nanoscale 2018, 10, 6684.
[2] Liao, T.-W.; Verbruggen, S. W.; Claes, N.; Yadav, A.; Grandjean, D.; Bals, S.; Lievens, P. Nanomaterials 2018, 8, 30.
[3] Liao, T.W; Yadav, A.; Ferrari, P.; Niu, Y.; Wei, X.W.; Vernieres, J.; Hu, K.-J.; Heggen , M.; Borkowski, R.D.; Palmer, R.E.; Laasonen, K.; Grandjean, D.; Janssens, E.; Lievens, P., submitted.
[4] Yadav, A.; Sanzone, G.; Najafishirtari, S.; Aghakhani, S.; Liao, T.W; Hu, K.-J.; Longo, A.; Yin, J.; Sels, B.; Grandjean, D.; Lievens, P., in preparation.
[5] Geerts, L.; Cosentino, S.; Liao, T.-W.; Yadav, A.; Lin, P.-C.; Zharinov, V. S.; Hu, K.-J.; Longo, A.; Pereira, L. M. C.; Grandjean, D.; Rongé, J.; Lievens, P.; Martens, J. A. Catalysis Today 2019.

Core-Shell nanoparticles towards functionality: Surface wetting, Hydrogen storage, Solar cells
Nowadays monometallic and bimetallic nanoparticles (NPs) have emerged as key materials for important modern applications in plasmonics, catalysis, biodiagnostics, energy storage, and magnetics. Consequently the control of NP structural motifs with specific shapes provides increasing functionality and selectivity for related applications. However, producing bimetallic NPs with well controlled structural motifs still remains a formidable challenge. In my talk I will address first the issues of a methodology for gas phase synthesis of bimetallic NPs with distinctively different structural motifs ranging at a single particle level from a fully mixed alloy to core–shell, to onion (multi-shell), and finally to a Janus/dumbbell with the same overall particle composition, as well as emphasis will be given to Hydrogen storage systems involving Mg-M (Cu, Ni, Ti). Furthermore, I will discuss recent results for Ge nanoparticles towards solar cells within the concept of band gap engineering of quantum dots. Finally, I will discuss issues related to roughness controlled (super) hydrophobicity on single nanometer length scale with metal/non-metal (Cu, C, GeSbTe) nanoparticles. Independent of the exact details, our approach to alter the wetting state and induce droplet trapping is straightforward without involving special micro/nano (hierarchical) structuring facilities but instead using direct single distinct nanoparticle deposition onto any type of surface (hydrophilic and/or hydrophobic). Details of the effect of airborne hydrocarbons on nanoparticle based nanostructured surfaces will be discussed.

Cluster (Nanoparticle) Beam Deposition: A Novel Route to the Solvent-Free Creation of Heterogeneous Catalysts
Size-selected nanoparticles (atomic clusters), deposited onto supports from the beam in the absence of solvents, represent a new class of model systems for catalysis research and possibly small-scale manufacturing of selective catalysts. To translate these novel and well-controlled systems into practical use, two major challenges have to be addressed.
(1) Very rarely have the actual structures of clusters been obtained from direct experimental measurements, so the metrology of these new material systems has to improve. The availability of aberration-corrected HAADF STEM is transforming our approach to this structure challenge [1,2]. I will address the atomic structures of size-selected Au clusters, deposited onto standard carbon TEM supports from a mass-selected cluster beam source. Specific examples considered are the “magic number clusters” Au20, Au55, Au309, Au561, and Au923. The results expose, for example, the metastability of frequently observed structures, the nature of equilibrium amongst competing isomers, and the cluster surface and core melting points as a function of size. The cluster beam approach is applicable to more complex nanoparticles too, such as oxides and sulphides [3].
(2) A second major challenge is scale-up, needed to enable the beautiful physics and chemistry of clusters to be exploited in applications, notably catalysis [4]. Compared with the (powerful) colloidal route, the nanocluster beam approach [5] involves no solvents and no ligands, while particles can be size selected by a mass filter, and alloys with challenging combinations of metals can readily be produced. However, the cluster approach has been held back by extremely low rates of particle production, only 1 microgram per hour, sufficient for surface science studies but well below what is desirable even for research-level realistic reaction studies. In an effort to address this scale-up challenge, I will discuss the development of a new kind of nanoparticle source, the “Matrix Assembly Cluster Source” (MACS) [4-6]. The results suggest cluster beam yields of grams per hour may ultimately be feasible; 10 mg scale has been demonstrated. Some practical applications [5,7,8] in heterogeneous catalysis (both gas and liquid phases), as well as electrocatalysis, will be presented, showing attractive activities and especially selectivities.
[1] Z.Y. Li et al, Nature 451 46 (2008).
[2] Z.W. Wang and R.E. Palmer, Phys. Rev. Lett. 108 245502 (2012).
[3] D. Escalera-Lopez, Y.B. Niu, J.L. Yin, K. Cooke, N.V. Rees, R.E. Palmer, ACS Catalysis 6 6008 (2016).
[4] P.R. Ellis et al, Faraday Discussions 188 39 (2016).
[5] R.E. Palmer et al. Acc. Chem. Res. 51 2296 (2018).
[6] R.E. Palmer, L. Cao and F. Yin, Rev. Sci. Instrum. 87 046103 (2016).
[7] R. Cai, P.R. Ellis, J.L. Yin, J. Liu, C.M. Brown, R. Griffin, G. Chang, D. Yang, J. Ren, K. Cooke, P.T. Bishop, W. Theis and R.E. Palmer, Small 14 1703734 (2018).
[8] J. Xu et al, ACS Appl. Energy Mater. 1 3013 (2018).

Nanoparticle Size, Shape, Support and Composition Effects in CO2 Conversion to Valuable Chemicals and Fuels
Tailoring the chemical reactivity of nanomaterials at the atomic level is one of the most important challenges in catalysis research. However, in order to achieve this elusive goal, we must first obtain fundamental understanding of the structural and chemical properties of these complex systems. In addition, the dynamic nature of the nanoparticle (NP) catalysts and their response to the environment must be taken into consideration. Despite the significant progress in experimental tools for NP characterization and theoretical NP modeling approaches, understanding the relation between intriguing properties of metal NPs (e.g., catalytic activity or unique thermodynamic characteristics) and their structure and surface composition is still a challenging task. The intrinsic complexity and heterogeneity of NPs, their interactions with the support, ligands and adsorbates, strong static disorder and anharmonic effects at NPs surface, or in situ transformations of their structure pose significant difficulties both for theoretical modeling and for the interpretation of experimental data. In this talk I will address some of these issues by bridging theoretical modeling (molecular dynamics (MD)) and experiments (extended X-ray absorption fine-structure spectroscopy (EXAFS)) via machine learning methods. Experimentally, the complexity of the real-world catalysts will be further addressed by taking advantage of a variety of cutting-edge complementary experimental methods (EC-AFM, EC-TEM, TPD, NAP-XPS, XAFS, MS/GC).
This talk will provide new insights into the thermal hydrogenation and electrocatalytic reduction of CO2. Important aspects that will be discussed are: (i) the design of size-and shape-controlled catalytically active NPs (Cu, Cu-Zn) on C, Al2O3, ZnO, ZnOAl, SiO2, and (ii) the investigation of structure/chemical state-reactivity correlations in situ and under realistic operando reaction conditions, i.e., at high pressure or under potential control. For example, time-dependent EXAFS data combined with neural network analysis will be used to gain insight into the structural evolution and brass alloy formation during the electrochemical reduction of CO2 over micellar CuZn NPs. Overall, our results are expected to open up new routes for the reutilization of CO2 through its direct conversion into valuable chemicals and fuels such as ethylene, methanol, ethanol, and propanol.

Mass-Selected Nanoparticles for Conversion of Sustainable Energy
In this presentation, I will give an overview of some our recent progress in making nanoparticles alloys and intermetallic compounds for catalysis, particularly in relation to conversion of sustainable energy [1]. We shall demonstrate how mass-selected nanoparticles synthesized, can be used to elucidate the activity for processes related to electrolysis and the reversible process in fuel cells. The electrochemical Oxygen Reduction Reaction, is the limiting reaction in Proton Exchange Membrane Fuel Cells. Here we have found entirely new classes of electro-catalysts by alloying Pt with lanthanides [2]. We have shown that it is possible to make mass-selected nanoparticles of these alloys with very good activities [3] and PtGd alloys [4]. We shall demonstrate that a similar approach can be used for studying size dependence and efficiency for catalysts related to water splitting and evaluate the scalability of scarce and expensive elements like Platinum [5] and Ruthenium [6]. Size dependence and isotope labelled experiments will be presented for NiFe nanoparticles for oxygen evolution under alkaline conditions [7]. Here we shall demonstrate a new principle for dynamic detection of gas evolution [8]. This has also been used to investigate electrochemical CO hydrogenation on mass-selected Copper nanoparticles to study the dynamical influence of surface oxygen on the selectivity for electrochemical methane/ethene production [9].
[1] Z. W Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov, T. F. Jaramillo, Science (2017) 355
[2] M. Escudero-Escribano, … I. E.L. Stephens, I. Chorkendorff, Science 352 (2016) 73.
[3] P. Hernandez-Fernandez, , …, I. Chorkendorff, Nature Chemistry 6 (2014) 732.
[4] A. Velázquez-Palenzuela…. I. Chorkendorff, J. Catal. 328 (2015) 297.
[5] E. Kemppainen, .. I. Chorkendorff, Energy & Environmental Science, 8 (2015) 2991.
[6] E. A. Paoli, F. … I. E.L Stephens, I. Chorkendorff, Chemical Science, 6 (2015) 190.
[7] C. Roy, …J. Kibsgaard, I. E. L. Stephens, and I. Chorkendorff; Nature Catalysis (2018) DOI: 10.1038/s41929-018-0162-x
[8] D. T. Bøndergaard, .. I Chorkendorff and P. C. K. Vesborg, Electrochem. Acta (2018) doi.org/10.1016/j.electacta.2018.02.060
[9] S. B. Scott, …. P. C. K. Vesborg, Jan Rossmeisl, and I. Chorkendorff, submitted (2018).

Formation and Emission Mechanisms of Ag, AuPd and AuAg Nanoclusters in Ar Matrix Assembly Cluster Source
There are different attempts to increase the production level of the nanoclusters to the industrial level. The Matrix Assembly Cluster Source (MACS) represents one of such opportunity by bridging conventional instruments for Cluster Beam Deposition (CBD) and the level of industrial production. The method is based on Ar+ ion sputtering of a pre-condensed Ar-metal matrix, typically Ag. Each Ar+ ion produces a collision cascade and initiating clustering of dispersed metal atoms is in the matrix. On a later stage, these clusters can be sputtered out. In my talk, I will mainly focus on the theoretical understanding of this process, giving only a general outline of the experimental work. Coming from the Accelerator laboratory, where the radiation effects are in the core of the research topics, I will give deeper insights on the processes developing in the matrix and leading to the sputtering of the clusters.
Our molecular dynamic simulations show that the cluster growth mechanism within the cryogenic matrix can be described as a “thermal spike-enhanced clustering”, which takes place in multiple sequential ion impact events. We describe the mechanism of emission of the metal nanocluster as forming first within the cryogenic matrix, and then emitting as a result of the simultaneous effects of interface boiling and spring force. We also investigate the dependence of cluster emission process on the incident angle of Ar+ ions. Using additionally the binary collision approximation simulation code, we analyze the position of the maximum deposited energy with respect to the position of the cluster and the entrance point of the ion. We find that the incidence angle strongly influences the emerging cluster flux, which is assigned to the spatial location of the deposited primary ion energy relative to the cluster into the matrix. We further study the formation of AgAu and PdAu nanoalloys. We also find that the phase segregation of PdAu nanoalloy is explained by the different melting points of Pd and Au.

Gas Phase Synthesized Electrocatalysts: From Proof of Concept to Application
Gas phase synthesis of nanoparticles (NPs) provides excellent control over size, morphology, crystallinity and NP-support interaction nanoparticles enabling us us in understanding and monitoring of structure- property relationship of nanocatalysts. We have successfully used the gas phase synthesis protocol for establishing proof of concept in single particle electrocatalytic activity and also for monitoring electronic NP-support interaction for enhanced electrocatalytic water splitting applications. Pd NP deposited on Mg thin films were exploited as nanoportals for charge transport and characterized extensively to study the individual electrocatalytic activities of this particles in ensemble. This work essentially proved the concept of studying individual particle activities in ensemble for the first time. Using the gas phase synthesis protocol we were also able to monitor the relative population of different crystallinities in Ru NP i.e. HCP and FCC. Since different crystalline structures of Ru NP results in different degree of electronic metal support interaction (EMSI) with CuO, controlling the relative population of different crystallinities also controls the EMSIs. This was extensively studied using various in situ spectroscopic and microscopic techniques. The electrochemical water splitting activities of Ru-CuO systems were also optimized using the control over EMSI. Hence both proof of concept and direct application in the field of electrocatalysis could be achieved by virtue of gas phase synthesis of nanoparticles.
[1] Datta et al. Single Nanoparticle Activities in Ensemble: a Study on Pd Cluster Nanoportals for Electrochemical Oxygen Evolution Reaction. J. Phys. Chem C 2019 (Ahead of Print)
[2] Porkovich et al. In Situ Observation of Metal to Metal Oxide Progression: A Study of Charge Transfer Phenomenon at Ru–CuO Interfaces. ACS Nano 2019, ( DOI: 10.1021/acsnano.9b06224)

High-Flux Ion Soft Landing for Preparation of Cluster Films and Supercapacitors
Historically, ion soft landing has been used as a highly-controlled method of surface modification to prepare relatively low coverage samples for fundamental studies in catalysis and surface science. Such low coverages are beneficial as they enable studies of essentially isolated species on supports where the effects of neighboring clusters and substrate-mediated interactions may be disregarded as negligible. In recent years, however, developments in ionization sources and mass spectrometry instrumentation have substantially increased the current of mass selected ions that may be delivered to surfaces. This new capability is taking soft landing in a new direction of high surface coverage research where unexpected phenomena, not observed before at lower coverages, are revealing themselves. Herein, we report a novel phenomenon by which macroscopic liquid-like layers with tunable self-organization properties form through accumulation of large numbers of stable ions of only one polarity on surfaces. Using a series of highly-stable and well-defined molecular anions with systematically varying internal charge distributions, we demonstrate a strong influence of the intrinsic properties of the ions, which are usually shielded by counterions in the condensed phase, on the properties of the soft landed layers. Characterization reveals that the intrinsically unstable films of anions on surfaces are stabilized by accumulation of neutral molecules which may also be used to tune the properties of the layers. High-flux soft landing also enables preparation of macroscopic operational devices such as supercapacitors from clusters with precise stoichiometry. These materials yield improved energy storage capacity and durability at the device scale. We describe how the composition of mixed-metal polyoxometalate clusters may be varied atom-by-atom to identify mixed-metal clusters with electronic properties that are ideally suited to electrochemical energy storage and increased stability.

Low-temperature catalysis of electron donation owing to strong electronic interaction between platinum cluster and silicon substrate
It has been found that O2 molecules are dissociated on a Pt cluster disk bound to a Si substrate, PtN/Si (N=10-60), at lower temperature by 150 K [1,2] than on the Pt(111) single-crystal surface [3], resulting in low-temperature CO oxidation. Considering the O2 dissociation is driven by electron transfer to anti-bonding molecular orbitals of O2, the cluster acts as a highly-efficient electron donor. Indeed, it has been found in our STM observation that electrons are accumulated at the sub-nano interface between PtN and the Si substrate [4,5] owing to strong electronic cluster-surface interaction. In this presentation, low-temperature dissociation of NO on the cluster disk is reported to show the universality of this cluster as well as in connection with catalytic NO reduction at low temperature, which is indispensable to clean environment in use of combustion engines particularly at high air-to-fuel ratios (lean combustion) having high fuel efficiency but emission of substantial nitrogen oxides, NOx,.
The size-selected cluster disks were prepared by cluster-impact [6] of size-selected cluster ions. The initiation temperature of the NO reduction was measured by means of temperature-programed desorption (TPD) of N2 produced, while its turnover-rate (TOR) by continuous gas-flow reactions [7]. It was observed that the NO reduction proceeds on PtN/Si at lower temperature by 70 K than on the Pt(100) single-crystal surface [8]. Its rate-determining step was found to be the NO dissociation, so that it is reliable that the NO dissociation on PtN/Si takes place at the lower temperature. Furthermore, the TOR measurements show high chemical selectivity such that only N2 but neither N2O nor NO2 are produced even with excess O2, mimicking the exhaust gas of the lean combustion.
It is concluded that the low-temperature catalysis of electron donation by PtN/Si has been demonstrated universally, considering the NO dissociation is also driven by the electron transfer to the anti-bonding molecular orbitals of NO. The resulting N adsorbates are combined each other to be N2 with high efficiency, but combination between NO and N/O adsorbates are negligible. It is promising that this activity of electron donation is derived from the accumulated electrons.
[1] H. Yasumatsu, Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry, ed. Klaus Wandelt (Editor-in-Chief), Elsevier, Volume 3.2: Clusters and Nanoparticles on Surfaces, pp. 477-489 (2018).
[2] H. Yasumatsu and N. Fukui, J. Phys. Chem. C 119, 11217 (2015).
[3] J. Yoshinobu and M. Kawai, J. Chem. Phys. 103, 3220 (1995).
[4] H. Yasumatsu, T. Hayakawa and T. Kondow, Chem. Phys. Lett. 487, 279 (2010).
[5] H. Yasumatsu, P. Murugan and Y. Kawazoe, Phys. Stat. Solidi B 6, 1193 (2012).
[6] H. Yasumatsu and T. Kondow, Rep. Prog. Phys. 66, 1783 (2003).
[7] H. Yasumatsu and N. Fukui, Catal. Sci. Technol. 6, 6910 (2016).
[8] Y. Ohno et al. Chem. Phys. Lett. 373, 161 (2003)

Building a computer that thinks like the brain using percolating nanoparticle networks
Recent progress in artificial intelligence and machine learning means that humans are now far inferior to computers at playing games like chess and go. However the brain is still much better than even the largest supercomputers at performing some types of tasks, such as pattern or image recognition.
This has motivated a worldwide effort to build brain-like, or “neuromorphic”, computers using a number of different approaches. I will review some of those approaches, which include the use of traditional silicon transistors to emulate neurons and synapses, and new solid-state devices which have synaptic and neuronal functionality.
I will then talk about how my group has attacked one of the key remaining challenges, which is to achieve truly brain-like networks using self-assembled nano-components. I will show that we have not only been able to build highly complex networks but that the dynamical signals within those networks are remarkably similar to those of the brain.

Size- and Composition-Selected Cluster Catalysts.
We are interested in exploring alloying as a means to improve the chemical and sinter stability of sub-nano cluster catalysts, formed by size-selected cluster deposition in UHV. In principle, mass selection can produce beams of size- and composition-selected clusters, however, for many combinations of metals, clean selection by mass is impractical except for clusters of just a few atoms. We have been exploring use of a two-step alternative. Mass-selected clusters of the first metal (e.g. Pt) are deposited by soft landing on the catalyst support of interest, and then atomic layer deposition (ALD) is used to deposit the second metal. By suitable choice of deposition conditions and reagents, we have found that the second metal can be deposited with high selectively, i.e., deposited only on the seed clusters. The number of atoms of the second metal per cluster appears to be determined by the number of binding sites on the seed cluster, and the size of the gaseous reagent used to carry the second metal.
The Pt-Sn/alumina and Pt-Sn/silica systems are good examples. Ptn are soft-landed on an alumina or silica supports, then exposed to H2, which adsorbs dissociatively on the clusters, but not on the oxide support. Exposure to SnCl4 leads to reaction with the H atoms, resulting in HCl desorption, and binding of SnClx to the clusters. The remaining Cl is removed by additional exposure to H2 with mild heating. The stoichiometry is ~1:1: for small Pt clusters where the seed clusters deposit as a single layers on the support, dropping significantly for larger seed clusters as they transition to 3D structures with fewer surface sites to bind SnClx.
The effect of tin alloying on ethylene binding and dehydrogenation is quite striking, as shown in the figure for the case of Pt-Sn/alumina catalysts based on Pt7 seed clusters. All samples were flashed to 700 K prior to ethylene exposure at 180 K. For pure Pt7, ethylene desorbs around 300 K (the lower temperature peak is from the alumina support), and a significant amount of dehydrogenation is observed at higher temperatures, leading to carbon deposition. If the experiment is repeated, the ethylene desorbs at lower temperatures, because the more stable binding sites are carbon-poisoned. For Pt7Sn~7, the ethylene binding energy is increased compared to Pt7. Nonetheless, there is substantially less dehydrogenation, and it can be seen that there is essentially no change in the ethylene binding behavior in the 2nd run, i.e. the clusters are quite stable. Theory is used to probe the alloy cluster geometric and electronic structures, and the binding mechanisms.
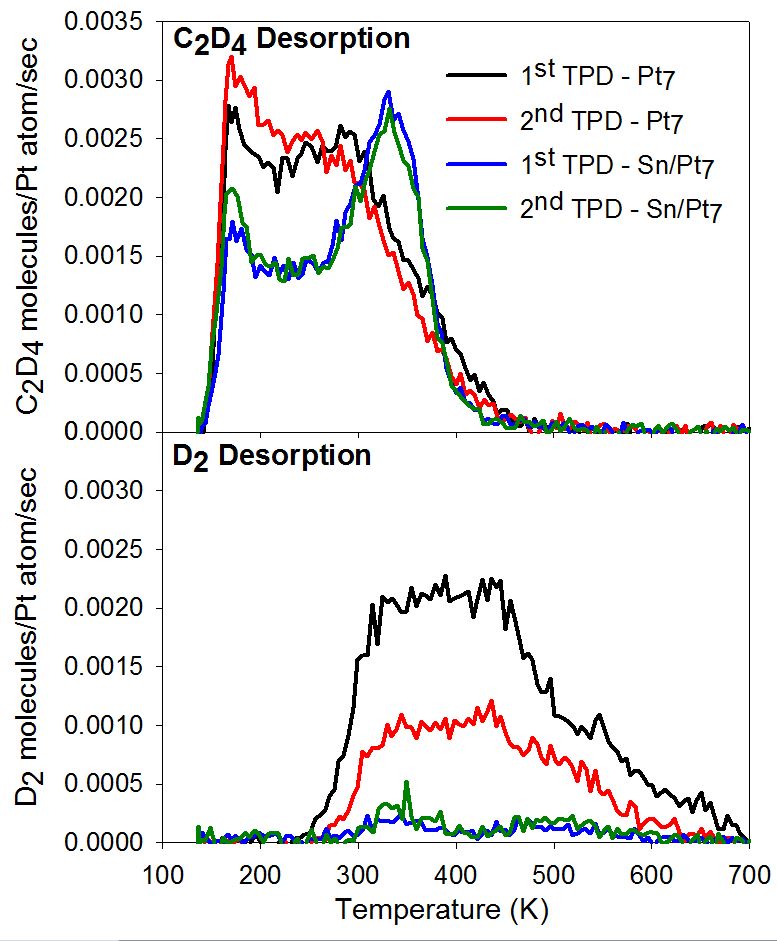
Figure 1: Effect of Sn-alloying on ethylene binding and dehydro-genation on Pt7/alumina

Modifying and Adapting Properties of Nanoparticles through Atomic Scale Computational Design
Core/shell metallic nanoparticles (NPs) and semiconductors nanostructures, in which different materials are involved, offer a way to relax the strain induced by the lattice parameter mismatch and create defect-free interfaces, owing to their large surface to volume ratio. Moreover, numerous structural phenomena related with metallic NPs such as coalescence, sintering, segregation and aggregation are frequently observed and modeled using Molecular Dynamics (MD) and Monte Carlo (MC) methods. In addition, NP-surface interaction involves a variety of fundamental phenomena, providing a unique system for studying electron transport mechanisms. On the other hand, semiconductor heterostructures offer the possibility of electronic property engineering and thus find their way in numerous optoelectronic applications. Ab initio calculations as well as interatomic potential based MD and MC simulations are employed to investigate the structural, thermal and electronic properties of a variety of core/shell nanoparticles and nanowires as well as of nanodisks embedded in nanowires. The band structures and charge transfer of the nanostructures are scrutinized in several cases through ab initio simulations of thousands of atoms.
By the use of atomistic simulations, materials analysis and design is implemented on extended defects as well as surfaces, interfaces and nanostructures at the atomic scale. Based on the aforementioned methodology technologically important cases are analyzed and elucidated.

The ability to precisely engineer well-defined nanoparticles and nanostructures in ever more complex configurations will allow increased functionality for nanoscale devices. The non-equilibrium inert gas condensation (IGC) process and the scale of the nanoparticles provide a route to create a wealth of atomic configurations, including amorphous structures, solid solutions in non-mixing systems, new crystal structures, and core/shell and core/frame gooemetries. These unique structures provide opportunities to explore and develop new materials, or for new or unique functionality. For example, novel crystal structures can form from parent non-equilibrium solid solutions, providing a feasible route to new materials. Core/shell and core/frame structures can show unique electronic or magnetic properties, which can be exploited in sensors or other devices. One example is core/shell Co@ZnO, where ZnO was formed either in the deposition chamber or upon exposure to atmosphere. Conductive atomic force microscopy on single particles displayed resistive switching behavior in the I-V measurements. These and other structures and properties of complex nanoparticles will be discussed.

Gas phase synthesis of clusters and nanoparticles using a scaled-up multi-magnetron gas aggregation source
The growing interest in gas phase synthesis of nanoparticles is motivated by different factors like the capacity to fabricate nanoparticles out-of-thermodynamic equilibrium process or the need of solvent-free methods that enable greener approaches for nanoparticle production [1]. Among different types of cluster sources [2], some years ago we developed a multi-magnetron GAS, called Multiple Ion cluster Source (MICS), to fabricate nanoparticles with controllable and tunable size, composition and structure [3,4]. More recently, we succeeded to scale-up this equipment using three independent magnetrons of 2” and a redesigned aggregation zone [5]. In this talk I will present details of the scaled-up MICS, the capacity to inject gases at different positions of the aggregation zone (i.e.: between the magnetron and the nozzle, in a region where the nanoparticles have been already started nucleating) avoiding poisoning of the target or the lateral entrances to make plasma monitoring. I will present some results obtained with metals and their interaction with oxygen as well as the fabrication of carbonaceous clusters and nanoparticles and their interaction with different gases as H2, CH4, C2H2 or O2 [6].
[1] R. E. Palmer, R. Cai, and J. Vernieres, Acc. Chem. Res., 51 (2018) 2296−2304.
[2] Gas-phase Synthesis of nanoparticles, WILEY‐VCH Verlag, 2017, Edited by Yves Huttel.
[3] L. Martínez, M. Díaz, E. Román, M. Ruano, D. Llamosa P. and Y. Huttel, Langmuir, 28 (2012) 11241.
[4] D. Llamosa P, M. Ruano, L. Martínez, A. Mayoral, E. Román, M. García-Hernández, Y. Huttel, Nanoscale, 6 (2014) 13483.
[5] L. Martínez, K. Lauwaet, G. Santoro, J.M. Sobrado, R.J. Peláez, V.J. Herrero, I. Tanarro, G. Ellis, J. Cernicharo, C. Joblin, Y. Huttel, J.A. Martín-Gago, Scientific Reports, 8 (2018) 7250.
[6] L. Martínez et al., Submitted.

Gas-phase synthesis of nanoparticles as witnessed by in situ diagnostics
Low-temperature plasma-based methods of the synthesis of nanoparticles (NPs) have been gaining considerable interest in recent years. Plasma polymerization of volatile organic precursors, rf magnetron sputtering of polymers and dc magnetron sputtering of metals have been adapted as powerful tools for the production of polymer, metal and heterogeneous NPs with tailored chemical composition and structure. The research has been pursuing numerous potential applications of the gas-phase synthesized NPs including optoelectronic energy conversion applications, electrocatalysts for fuel cells, new plasmonic materials superior to Au and Ag, electroluminescence, macromolecular self-assembly, supramolecular chemistry and drug delivery. Significant progress has been achieved in this area, although little experimental evidence has been collected about the processes occurring during the NP nucleation and growth.
This contribution investigates the mechanisms of the NP formation in the discharge with the focus set on in situ diagnostics techniques involving Small-Angle X-ray scattering. The development of a so-called capture zone is described that is responsible for the formation of large (up to 90 nm) NPs in the vicinity of the magnetron target. The time-resolved measurements indicate that the majority of the NPs are charged and influenced by electrostatic interactions. Part of the NPs become expelled from the capture zone both towards the magnetron as well as in an outward direction. The larger NPs become continuously lost on the walls during their way from the magnetron to the exit orifice so that only smaller NPs constitute the final deposit in the deposition chamber.
Acknowledgements
This work was supported by the grant GAČR-17-12994S from the Grant Agency of the Czech Republic.

Gas phase synthesis of nanoparticles and particulate nanocomposites for functional applications
Nanoparticles have hosts of applications due to the functional properties arising from their nanoscale dimensions. Moreover, they provide the basis for particulate nanocomposites consisting of metal nanoparticles in a dielectric organic or ceramic matrix. In particular, highly filled particulate nanocomposites exhibit unique functional properties due to the interaction between nanoparticles on the nanoscale. Fabrication of highly filled particulate nanocomposites hence requires nanoscale control of the particle separation. The present talk demonstrates how vapor phase deposition techniques can be employed for the synthesis of nanoparticles and particulate nanocomposites and for tailoring the resulting properties. Vapor phase deposition, inter alia, allows excellent control of the metallic filling factor and its depth profile as well as the incorporation of alloy nanoparticles with well-defined composition. The nanoparticles are synthesized by means of high-rate gas aggregation cluster sources, which also allows independent control of filling factor and size of the embedded nanoparticles. Formation of plasmonic nanoparticles can be monitored in situ via UV-vis spectroscopy. We also demonstrate in situ control of the composition of alloy nanoparticles and the ability to fabricate multiple core-shell particles. Examples of fabricated nanocomposites range from plasmonic meta-materials including superabsorbers through photoswitshable devices to memristors. Moreover, we will present a novel process for photocatalytic growth of Au nanostructures.

Preparation of Liquid Suspensions of Nanoparticles for Medical Applications By Cluster Beam Deposition
The flexibility of gas-phase synthesis for the production of nanoparticles with designed properties is well known. It offers good control of particle size, flexible choice of material and the ability to produce complex internal structures such as alloy, core-shell and Janus particles. All this can be done within an ultra-high vacuum (UHV) source in which the nanoparticles can either be kept free of oxides or oxidised in a fully controlled manner. This versatility facilitates the design of nanoparticles with optimised performance in applications and this is especially important for medical applications. In these, some exciting possibilities, such as magnetic nanoparticle hyperthermia treatment of cancer, are limited by the performance of currently available oxide nanoparticles produced chemically. A technical problem with using cluster beam synthesised nanoparticles in medical applications however is how to get the particles from a UHV environment into water or other liquids without converting them to oxide.
This talk will present a method for achieving this that was invented at the University of Leicester in the UK[1] and is being further developed at UCLM in Spain. The nanoparticles formed in a free beam in UHV are deposited into a substrate maintained at 77K and water is injected as a molecular beam onto the same substrate. The nanoparticles are thus embedded in an ice layer maintained at 77K whose vapour pressure is ~10-14 hPa. Thus UHV can be maintained throughout the source and the nanoparticles can be produced as normal with all the flexibility described above. When the deposition is complete, the deposition chamber is isolated and the ice containing the nanoparticles is melted to produce the hydrosol. The talk will also discuss remedies for other problems such as how to prevent agglomeration of the nanoparticles and also the performance of cluster-beam produced hydrosols in magnetic nanoparticle hyperthermia and in diagnostic imaging methods such as MRI and MPI.
[1] Preparation of Hydrosol Suspensions of Elemental and Core-Shell Nanoparticles by Co-Deposition
with Water Vapour from the Gas-Phase in Ultra-High Vacuum Conditions, C. Binns, P. Prieto, S. Baker, P. Howes, R. Dondi, G. Burley, L. Lari, R. Kröger, A. Pratt, S. Aktas and J. K. Mellon, J. Nanoparticle Res. 14 (2012) 1136.

Tailored thermal transport in nanostructures and nanostructured materials
With the rapid evolution of materials elaboration a series of nanostructures and nanostructured material like phononic-like crystals or nanowire networks and nanocomposites are easily fabricated today. In general, decreasing dimensionalities of materials drives to the decrease of their thermal conductivity compared to their bulk state, due to phonon confinement and boundary scattering. New physical phenomena are observed in such structures as ballistic phonon transport, phonon focusing, phonon tunneling, coherence effects, thermo-hydo-dynamic flow, etc. Such phenomena will be presented in several nanometric systems mainly extracted from Molecular Dynamics Simulations and in some cases compared with available experimental measurements. Here, several structures will be discussed as phononic like crystals, crystalline/amorphous nanocomposites, 2D and 3D nanowires networks (figure). The purpose is to appraise the impact of surface roughness, amorphous phase integration, diameter modulation as well growth direction on the thermal conductivity.

Figure 1: 2D and 3D nanowire networks. Simulation cell containing one node for (a) 2D and (b) 3D networks, while (c), (d) represent the global modeled systems thanks to periodic boundary conditions. The green surfaces represent the total cross section of each system when considering the TC in the direction perpendicular to these surfaces.

X-ray diffraction imaging of nanoparticles in gas-phase
Most of our high-resolution imaging methods have to compromise between temporal or spatial resolutions, similar to a pinhole camera. If the entrance pinhole is very small, the resulting image is crisp, but requires a long exposure and thus, is less suitable to capture moving objects. One can increase the pinhole diameter and thus, increase the temporal resolution, but this comes at a cost of the sharpness of the image.
Of course, our microscopic tools have greatly evolved over time, but this original problem still limits our capabilities to observe fast processes at the nanoscale. Examples include chemical and catalytic reactions, nucleation dynamics and growth of nanoparticles, and other phenomena which are short-lived. One idea to overcome this obstacle is to use an illumination source, which is capable of producing intense short wavelengths radiation such as X-rays within very short exposure times.
X-ray Free Electron Lasers (FELs), such as the LCLS at SLAC, are capable of producing very bright bursts of coherent X-rays within a few femtoseconds. This large-scale technique offers unique opportunities to visualize transient processes as “frozen” in time via coherent X-ray diffractive imaging. I will present an overview over state-of-art nanoparticle imaging experiments with X-ray FELs, and discuss current challenges and opportunities. Our recent study with sub-fs short FEL pulses indicates, that we can achieve nm-scale resolution within a single 0.5 femtosecond exposure in images of single Xe ice nanoparticles. The data set also suggests that we might soon push the resolution limits even further by exploiting ionic resonances.
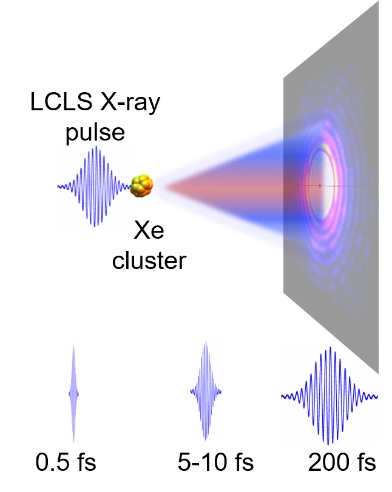
[1] Neutze, R., et al. Nature 406, 752–757 (2000), Loh, N. D. et al. Nature 486, 513–517 (2012), Barke, I. et al. Nat. Com- mun. 6, 6187 (2015), Gomez, L. F. et al. Science 345, 906–909 (2014), van der Schot, G. et al. Nat. Commun. 6, 5704 (2015), Seibert, M. M. et al. Nature 470, 78–81 (2011) Gorkhover, T. et al. Nat. Photon.10, 93–97 (2016)

Metal-Atom Encapsulating Silicon Cage Superatoms; M@Si16
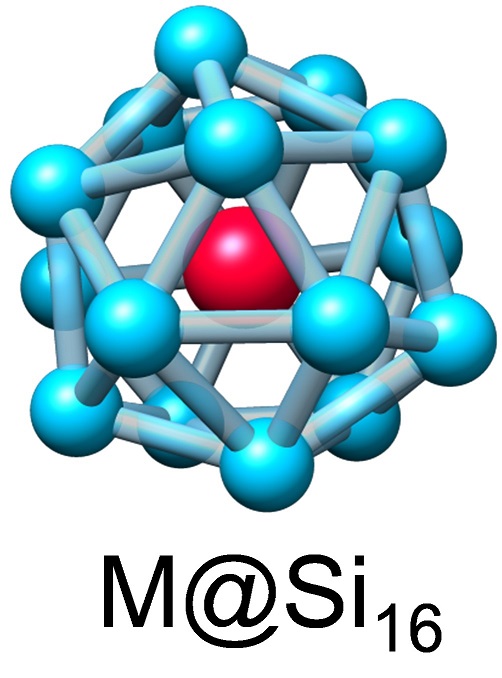
Since well-established function miniaturization of silicon (Si) devices with photolithography has almost reached its technological limit, it is urgent to explore new Si based low-dimensional functional nanomaterials (e.g. Si nanodots, nanowires, nanosheets) with bottom-up technologies utilizing physicochemical synthetic methods in the gas and liquid phases. Among the Si based low-dimensional functional nanomaterials, Si nanosheet of silicene, a counter part of graphene, is a two-dimensional (2D) Si nanomaterial, exhibiting high in-plane electric conductivity. Since the Si atom, unlike the C atom, generally prefers to sp3 hybridization, sp2-like Si-Si bonds should be formed to make the 2D flat silicene. However, the 2D silicene on a metal surface is non-flat due to the structural deviation from the sp2 conformation. By analogy with C60 fullerene, alternatively, a rounded silicene can be expected to be a caged surface consisting of sp2-Si.
We have found a periodic family of caged Si species as M@Si16 superatoms based on mass spectrometry, where halogen-like (Sc@Si16–), rare gas-like (Ti@Si16(0)), and alkali-like (V@Si16+, Ta@Si16+) superatoms have been demonstrated by the group-3, -4, and -5 atom encapsulations, respectively [1]. They complete their superatomic orbtal closure for the same number of valence electrons (68e), where 64 and 4 electrons come from the 16 Si atoms and the central M atom including charged states. Beyond the surface immobilization of the M@Si16 superatoms [2-4], we have developed a large-scale synthesis method for M@Si16 (M = Ti and Ta) by scaling up the clean dry-process with a high-power impulse magnetron sputtering (nanojima®) and by a direct liquid embedded trapping (DiLET) method [5]. The spectroscopic results reveal that the structures of isolated M@Si16 superatoms are the metal-encapsulating tetrahedral Si-cage (METS) consisting of sp2-Si atoms [6].
[1] K. Koyasu, M. Akutsu, M. Mitsui, and A. Nakajima, J. Am. Chem. Soc. 2005, 127, 4998.
[2] M. Nakaya, T. Iwasa, H. Tsunoyama, T. Eguchi, and A. Nakajima, Nanoscale 2014, 6, 14702.
[3] M. Shibuta, T. Ohta, M. Nakaya, H. Tsunoyama, T. Eguchi, and A. Nakajima, J. Am. Chem. Soc. 2015, 137, 14015.
[4] M. Shibuta, T. Kamoshida, T. Ohta, H. Tsunoyama, and A. Nakajima, Comm. Chem. 2018, 1, 50.
[5] H. Tsunoyama, H. Akatsuka, M. Shibuta, T. Iwasa, Y. Mizuhata, N. Tokitoh, and A. Nakajima, J. Phys. Chem. C 2017, 121, 20507.
[6] H. Tsunoyama, M. Shibuta, M. Nakaya, T. Eguchi, and A. Nakajima, Acc. Chem. Res. 2018, 51, 1735.

Nanoparticles by Design: a short biography
Cluster beam deposition (CBD) is a term that collectively describes various physical methods of nanoparticle synthesis by nucleation and growth from a supersaturated atomic vapour. It provides a solvent- and effluent-free method to design monodisperse multifunctional nanoparticles with tailored characteristics that can be subsequently deposited on a desired substrate or device in the soft-landing regime under ultra-high vacuum.
In this talk, I will explain the main mechanisms that control the basic properties of individual nanoparticles such as size, shape, or chemical ordering, based on various setups of CBD sources. Moving to a coarser scale, I will bring up examples where larger structures can be designed using nanoparticles as their functional building blocks.
To date, CBD faces two main limitations that need to be overcome for real-world applications: (i) limited yield, and (ii) precise structural control. The main thesis of this talk is that both challenges can be tackled by appropriate instrumentation upgrade, and by in-depth theoretical understanding of both the thermodynamics and kinetics of nucleation & growth. To this end, atomistic computer modelling can be an invaluable tool, complementing experimental fabrication or guiding future source design.



