FY2011 Annual Report
Optical Neuroimaging Unit
Professor Bernd Kuhn


Abstract
In FY 2011 the Optical Neuroimaging Unit almost completed the lab setup including two two-photon setups for in vivo imaging. One of the microscopes was acquired with generous support by a Kakenhi Grant to Kenji Doya in collaboration with the Optical Neuroimaging Unit. First research projects started focusing on brain imaging in vivo and in vitro. An additional project investigates the pH in protein crystals.
1. Staff
- Dr. Christopher J. Roome, Researcher
- Dr. Marylka Y. Uusisaari, Researcher
- Dr. Akihiro Funamizu, Researcher (shared with Doya Unit)
- Ryo Shiroishi, Graduate student (shared with Doya Unit)
- Dr. K. Kumkum Hossain, Research Assistant
- Hiroko Chinone, Research Administrator
2. Collaborations
- Theme: Investigation of model-based decision making by two-photon microscopy
- Type of collaboration: Joint research
- Researchers:
- Professor Dr. Kenji Doya, Neural Computation Unit, OIST
- Dr. Akihiro Funamizu, Neural Computation Unit, OIST
- Ryo Shiroishi, Neuroal Computation Unit, OIST
- Theme: Two-photon imaging in the lateral geniculate nucleus
- Type of collaboration: Joint research
- Researchers:
- Dr. Sigita Augustinaite, University of Oslo, Norway
- Professor Dr. Paul Heggelund, University of Oslo, Norw
- Theme: Combinatorial genetic approach for cell type specific expression of fluorescent calcium indicator proteins
- Type of collaboration: Joint research
- Researchers:
- Professor Dr. Ilker Ozden, Brown University, U.S.A.
- Yulia Lampi, Princeton University, U.S.A.
- Dr. Mazahir T. Hasan, Max-Planck-Institute for Medical Research, Germany
- Professor Dr. Samuel S.-H. Wang, Princeton University, U.S.A.
- Theme: pH measurement within protein crystals
- Type of collaboration: Joint research
- Researchers:
- Dr. Klaus M. Seemann, Peter Grunberg Institute, Research Center Julich, Germany
- Dr. Reiner Kiefersauer, Proteros biostructures GmbH, Martinsried, Germany
- Prof. Dr. Uwe Jacob, Westend-Innovation GmbH, Munchen, Germany
3. Activities and Findings
3.1 In Vitro and in vivo brain imaging
In FY2011 we almost completed the setup of the imaging lab. Also most of the techniques for surgery and imaging were established and tested. Behavioral training and virus injections began.
3.2 An amplified promoter system for targeted expression of calcium indictor proteins in the cerebellar cortex
In collaboration with Princeton University we developed an adeno-associated virus (AAV) based method to specifically target neuronal subtypes in the cerebellum. We are able to specifically label exclusively interneurons or Purkinje cells.
Together with our previously developed virus labeling cerebellar glia cells (Fig. 1, Kuhn et al., 2011d) we can now target almost all cell types of the cerebellar cortex for Ca2+ imaging and in the future also optogenetics.
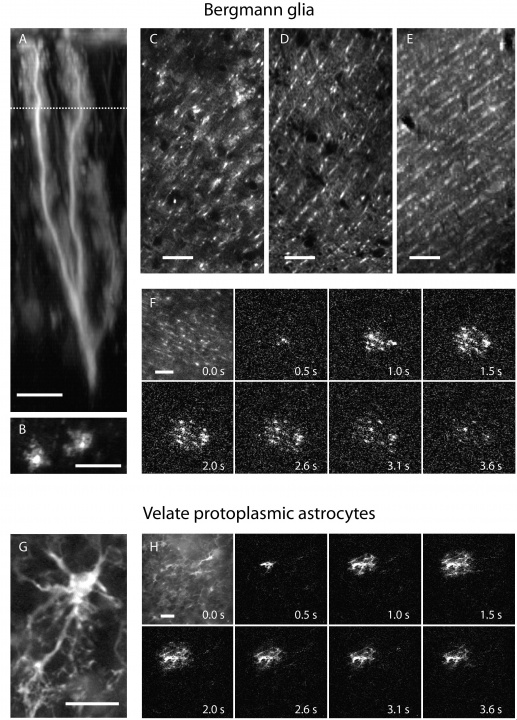
Figure 1:Astrocytes of the cerebellar cortex generate transglial calcium waves in vivo. (A) Bergmann glia cell expressing a fusion protein of the FCIP G-CaMP2 and DsRed under the CMV IE promoter after adenoviral gene transfer. (B) The use of FCIP also allows imaging of glial microdomains when cells are sparsely infected or (C) cell populations at higher infection rates which are almost comparable to (D) transgenic mice expressing GFP under the Bergmann glia-specific GFAP promoter. (E) Multicell bolus loading (MCBL) with the synthetic calcium dye fluo-5F/AM shows preferential Bergmann glia labeling if injected superficially into the molecular layer of the cerebellar cortex. (F) In vivo Bergmann glia show radially expanding transglial calcium waves measured with G-CaMP2. The first image shows resting fluorescence; following images show fluorescence changes relative to the first image. (G) Velate protoplasmic astrocyte of the granule cell layer expressing G-CaMP2 after infection by recombinant adenovirus. (H) Velate astrocytes also generate transglial calcium waves in vivo. The first image shows resting fluorescence; following images fluorescence changes relative to the first image. Scale bar in all panels, 20 µm.
3.3 Two-photon imaging in the lateral geniculate nucleus
After the installation of a new two-photon microscope at Oslo University calcium imaging in brain slice was performed.
3.4 pH measurement within protein crystals
The pH is one of the key parameters governing protein conformation and activity. Our current mechanistic understanding of cellular and molecular biology, however, is based on protein structures obtained from crystals. In protein crystals the pH is unknown and so the protein structures might be altered by an unexpected pH in the crystal. We are developing a new method to satisfy this demand for an experimental access to the crystal pH.
4. Publications
4.1 Journals
1. B. Kuhn, T.M. Hoogland, and S.S.-H. Wang (2011a) Injection of Recombinant Adenovirus for Delivery of Genetically Encoded Calcium Indicators into Astrocytes of the Cerebellar Cortex CSH Protocols 2011(10):pdb.prot065797
2. B. Kuhn, T.M. Hoogland, and S.S.-H. Wang (2011b) Cerebellar Craniotomy for In Vivo Calcium Imaging of Astrocytes CSH Protocols 2011(10):pdb.prot065805
3. T.M. Hoogland, B. Kuhn, and S.S.-H. Wang (2011c) Preferential Loading of Bergmann Glia with Synthetic Acetoxymethyl Calcium Dyes CSH Protocols 2011(10):pdb.prot065813
4.2 Books and Other One-Time Publications
1. B. Kuhn, T.M. Hoogland, and S.S.-H. Wang (2011d) In vivo calsium imaging of cerebellar astrocytes with synthetic and genetic indicators. In: Imaging in Neuroscience , 2nd Edition. F. Helmchen, A. Konnerth, and R. Yuste (Editors), CSHL Press, p. 707-720
4.3 Oral and Poster Presentations
1. X. R. Sun, Y. Lampi, T. Friling, E.R. Schneider, B. Kuhn, S. S.-H. Wang (2011) Improved tracking of neuronal activity using faster and lower-affinity variants of G-CaMP Society for Neuroscience Meeting, Washington, U.S.A., November, 2011
2. B. Kuhn Calcium imaging in the cerebellum of awake mice with genetically encoded indicators. OIST-University of Ryukyus Interchange, OIST Main Campus, Onna, Okinawa, June 17, 2011
5. Intellectual Property Rights and Other Specific Achievements
5.1 The Category or Type of Funding, like External Funding, Awards, etc.
Kakenhi grant (Grant-in Aid for Scientific Research on Innovative Areas) in collaboration with Kenji Doya, OIST
6. Meetings and Events
6.1 Workshop: Outlook for the Next Generation Neuroscience
- Date: March 05-07, 2012
- Venue: Nara Institute of Science and Technology, Nara, Japan
- Organizers: Professor S. Komai and Professor Y. Sakumura, Nara Institute of Science and Technology
- Speakers:
- Bernd Kuhn (OIST)
- Ali Cetin (Salk Institute)
- Albert Lee (Janelia farm)
- David Odde (University of Minnesota)
6.2 Workshop: Okinawa Computational Neuroscience Course 2011
- Date: June 13-30, 2011
- Venue: OIST Seaside House
- Organizers: Drs. E. De Schutter, K. Doya, K. Stiefel, J. Wickens
- Speaker (among others): Bernd Kuhn
7. Other
Nothing to report.



