Publications
[42] Hao Zhao, Rafael Muñoz-Mármol, Liliia Moshniaha, Qiqi Yang, Mischa Bonn, Xiaomin Liu, Ryota Kabe, Giuseppe Maria Paterno, Akimitsu Narita.
Acid-induced fluorescence enhancement of piperazinylphenyl-substituted nanographene. Chemical Communications, 60, 14645–14648, Issue 98, 21 December 2024, https://doi.org/10.1039/D4CC04926H
[41] Yutong Hu, Xuande Yang, Zetian Huang, Qiyan Xie, Yuhuan Chen, Yanqiong Zheng, Ryota Kabe, Daqing Zhang, Jinhai Huang, Yi Qu, Zhiyun Zhang.
D-A-D-type bipolar hosts incorporating N-phenylcarbazole and quinoline at various connection sites with high electron mobility for red electrophosphorescence.
Synthetic Metals, Volume 308, November 2024, 117712, https://doi.org/10.1016/j.synthmet.2024.117712
[40] Yanqiong Zheng, Qingyu Zhang, Xuande Yang, Yuhuan Chen, Bingjia Zhao, Huadong Zheng, Xifeng Li, Ryota Kabe, Longlong Chen.
Performance improvement of blue thermally activated delayed fluorescence organic light emitting diodes via in-situ fabricated honeycomb porous polystyrene pattern.
Optics and Lasers in Engineering, Volume 177, June 2024, 108137, https://doi.org/10.1016/j.optlaseng.2024.108137
[39] Tang X*, Xie M, Lin Z, Mitrofanov K, Tsagaantsooj T, Lee YT*, Kabe R, Sandanayaka ASD, Matsushima T, Hatakeyama T, Adachi C.
A Rigid Multiple Resonance Thermally Activated Delayed Fluorescence Core Toward Stable Electroluminescence and Lasing.
Angew Chem Int Ed Engl. Volume 63, Issue 2 (2024) https://doi.org/10.1002/anie.202315210.
[38] Lin Z, Li M, Yoshioka R, Oyama R, Kabe R*.
Oxygen-Tolerant Near-Infrared Organic Long-Persistent Luminescent Copolymers.
Angew Chem Int Ed Engl. e202314500 (2023) https://doi.org/10.1002/anie.202314500.
[37] Wu T, Xu X, Ono LK, Guo T, Mariotti S, Ding C, Yuan S, Zhang C, Zhang J, Mitrofanov K, Zhang Q, Raj S, Liu X, Segawa H, Ji P, Li T, Kabe R, Han L, Narita A*, Qi Y*.
Graphene-Like Conjugated Molecule as Hole-Selective Contact for Operationally Stable Inverted Perovskite Solar Cells and Modules.
Adv Mater. Volume35, Issue21 (2023) https://doi.org/10.1002/adma.202300169.
[36] Tan J, Xu X, Liu J, Vasylevskyi S, Lin Z, Kabe R, Zou Y, Müllen K, Narita A*, Hu Y*.
Synthesis of a π-Extended Double [9]Helicene.
Angew Chem Int Ed Engl. Volume 62, Issue 18 (2023) https://doi.org/10.1002/anie.202218494.
[35] Zhang, C.; Mariotti, S.; K. Ono, L.; Ding, C.; Mitrofanov, K.; Zhang, C.; Yuan, S.; Ji, P.; Zhang, J.; Wu, T.; Kabe, R.; Qi, Y.*
A Hole Injection Monolayer Enables Cost-Effective Perovskite Light-Emitting Diodes.
Journal of Materials Chemistry C 11, 2851–2862 (2023). https://doi.org/10.1039/D2TC05491D.
[34] Xu, X.; Serra, G.; Villa, A.; Muñoz-Mármol, R.; Vasylevskyi, S.; Gadea, M.; Lucotti, A.; Lin, Z.; G. Boj, P.; Kabe, R.; Tommasini, M.; Á. Díaz-García, M.; Scotognella, F.; Maria Paternò, G.*; Narita. A.*
Synthesis of Zigzag- and Fjord-Edged Nanographene with Dual Amplified Spontaneous Emission.
Chemical Science, 13, 13040–13045 (2022). https://doi.org/10.1039/D2SC04208H.
[33] Wu, T.; K. Ono, L.; Yoshioka, R.; Ding, C.; Zhang, C.; Mariotti, S.; Zhang, J.; Mitrofanov, K.; Liu, X.; Segawa, H.; Kabe, R.; Han, L.; Qi, Y.*
Elimination of Light-Induced Degradation at the Nickel Oxide-Perovskite Heterojunction by Aprotic Sulfonium Layers towards Long-Term Operationally Stable Inverted Perovskite Solar Cells.
Energy & Environmental Science 15, 4612–4624 (2022). https://doi.org/10.1039/D2EE01801B
[32] Jinnai, K., Kabe, R.*, Lin, Z., Adachi, C.*
Organic long-persistent luminescence stimulated by visible light in p-type systems based on organic photoredox catalyst dopants.
Nat. Mater. 21, 338–344 (2022) DOI:10.1038/s41563-021-01150-9
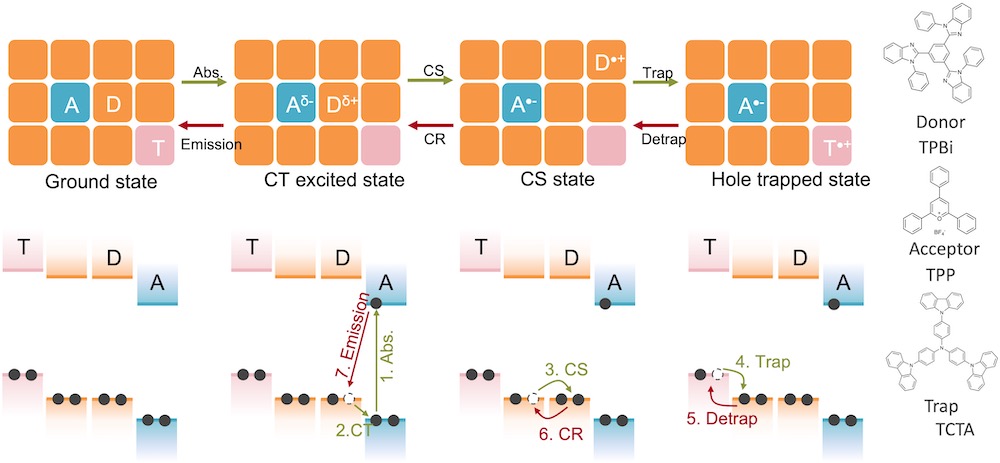
[31] Paternò, G. M., Chen, Q., Muñoz-Mármol, R., Guizzardi, M., Bonal, V., Kabe, R., Barker, A. J., Boj, P. G., Chatterjee, S., Ie, Y., Villalvilla, J. M., Quintana, J. A., Scotognella, F., Müllen, K., Díaz-García, M. A.*, Narita, A.*, Lanzani, G.*
Excited states engineering enables efficient near-infrared lasing in nanographenes.
Mater. Horizons 9, 393-402 (2022) DOI:10.1039/D1MH00846C
[30] Sakurai, M., Kabe, R.*, Fuki, M., Lin, Z., Jinnai, K., Kobori, Y., Adachi, C., Tachikawa, T.*
Organic photostimulated luminescence associated with persistent spin-correlated radical pairs
Commun. Mater. 2, 74 (2021) DOI:10.1038/s43246-021-00178-3
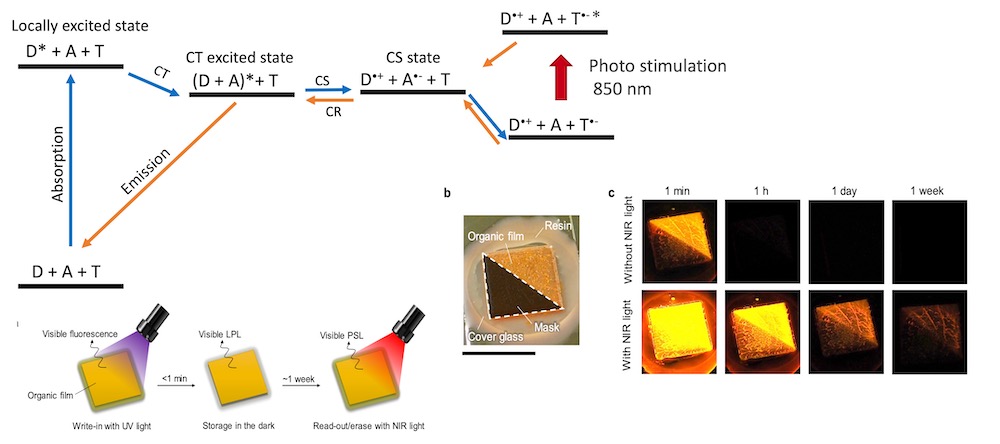
[29] Tan, S., Jinnai, K., Kabe, R.*, Adachi, C.*
Long-persistent luminescence from an exciplex-based organic light-emitting diode
Adv. Mater. 2008844 (2021) DOI:10.1002/adma.202008844
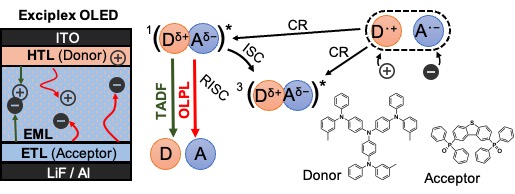
[28] Jinnai, K., Nishimura, N., Adachi, C.*, Kabe, R.*
Thermally activated processes in an organic long-persistent luminescence system
Nanoscale 13, 8412–8417 (2021) DOI:10.1039/D0NR09227D
[Nanoscale Emerging Investigators 2021]
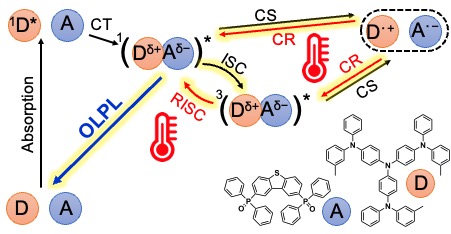
[27] Li, W., Li, Z., Si, C., Wong, M.Y., Jinnai, K., Gupta, A.K., Kabe, R., Adachi, C., Huang, W., Zysman‐Colman, E.*, Samuel, I.D.W.*:
Organic long‐persistent luminescence from a thermally activated delayed fluorescence Compound
Adv. Mater. 32, 2003911 (2020). DOI:10.1002/adma.202003911
[26] Nishimura, N., Lin, Z., Jinnai, K., Kabe, R.*, Adachi, C.*
Many exciplex systems exhibit organic long‐persistent luminescence
Adv. Funct. Mater. 30, 2000795 (2020). DOI:10.1002/adfm.202000795
[Inside front cover]
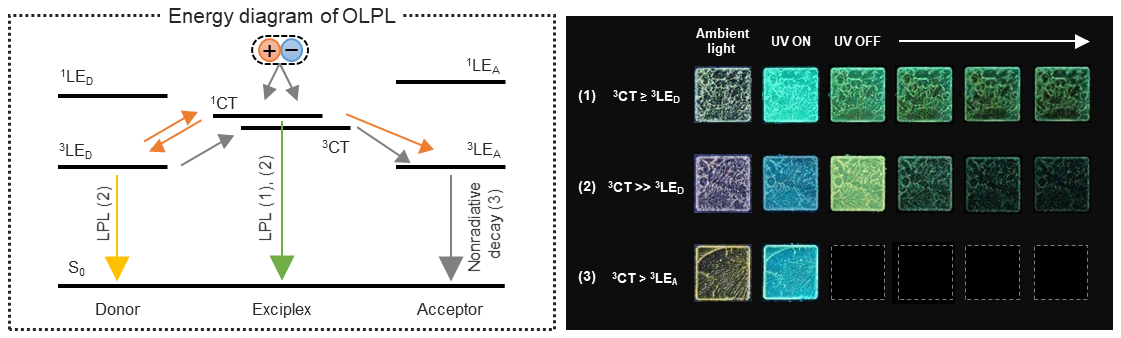
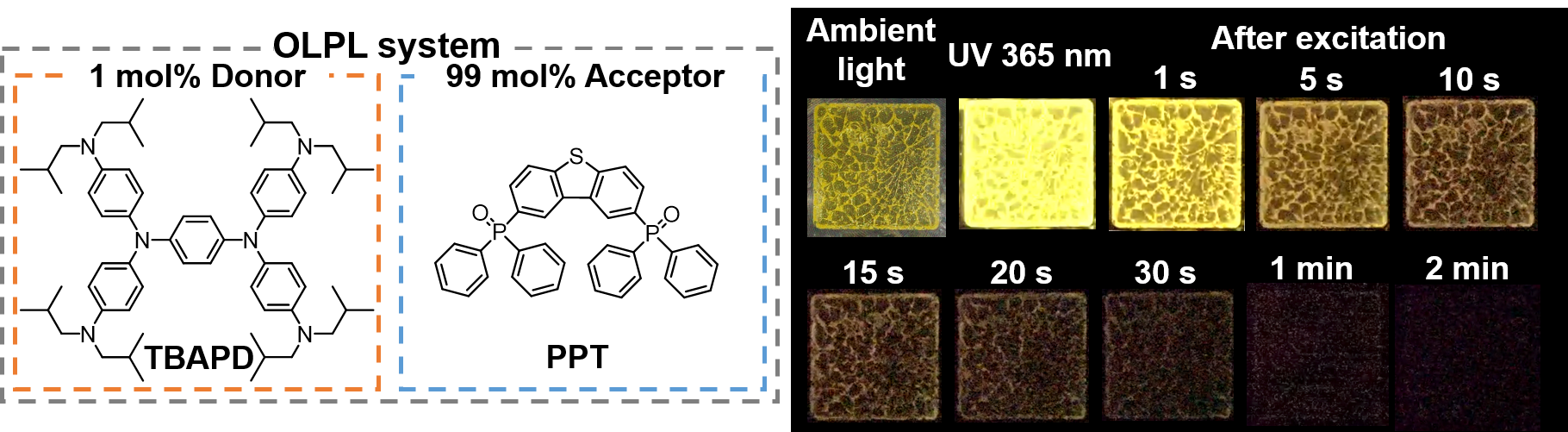
[25] Lin, Z., Kabe, R.*, Adachi, C.*
Orange organic long-persistent luminescence from an electron donor/acceptor binary system
Chem. Lett. 49, 203–206 (2020). DOI:10.1246/cl.190823
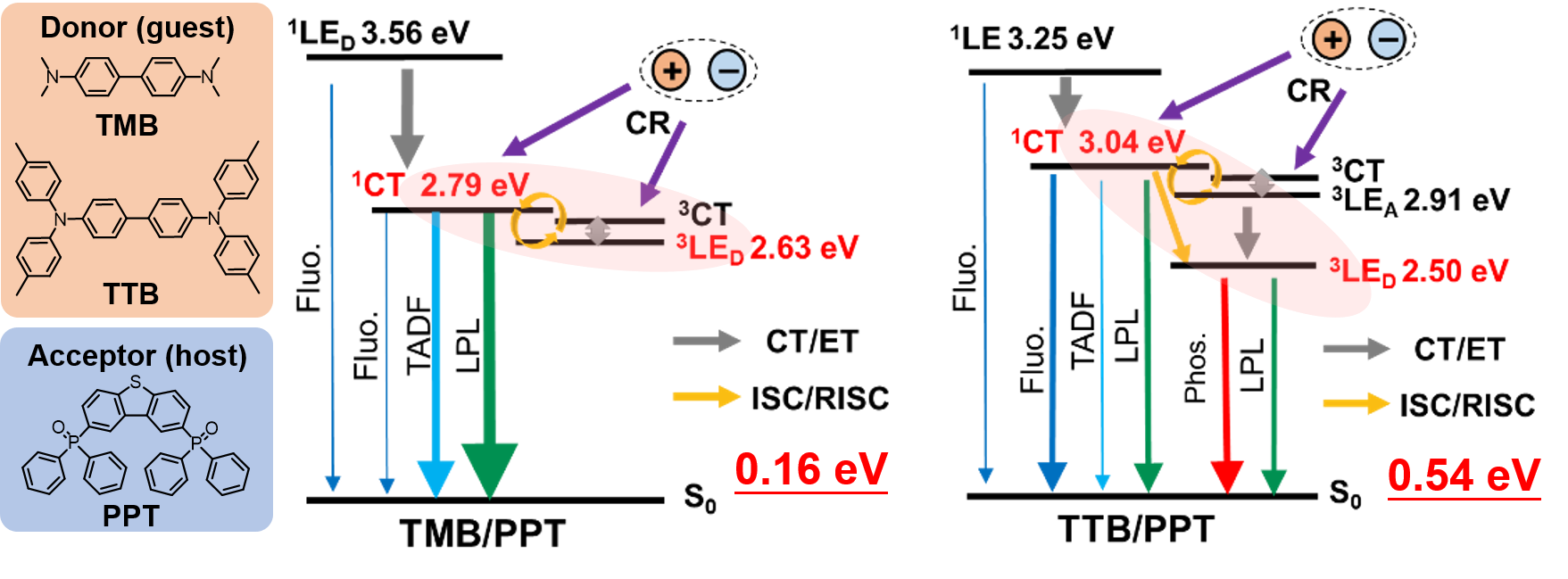
[24] Lin, Z., Kabe, R.*, Wang, K., Adachi, C.*
Influence of energy gap between charge-transfer and locally excited states on organic long persistence luminescence
Nat. Commun. 11, 191 (2020). DOI:10.1038/s41467-019-14035-y
[23] Jinnai, K., Nishimura, N., Kabe, R.*, Adachi, C.*
Fabrication-method independence of organic long-persistent luminescence performance
Chem. Lett. 48, 270–273 (2019). DOI:10.1246/cl.180949
[22] Mieno, H., Kabe, R.*, Allendorf, M.D., Adachi, C.*
Thermally activated delayed fluorescence of a Zr-based metal–organic framework
Chem. Commun. 54, 631–634 (2018). DOI:10.1039/C7CC08595H
[21] Xia, Z.*, Kabe, R., Liscio, A., Kovtun, A., Treossi, E., Feng, X., Palermo, V.*
Graphene-pyrene nanocomposites obtained using azide chemistry
J. Nanosci. Nanotechnol. 18, 1290–1295 (2018). DOI:10.1166/jnn.2018.15254
[20] Mieno, H., Kabe, R.*, Adachi, C.*
Reversible control of triplet dynamics in metal-organic framework-entrapped organic emitters via external gases
Commun. Chem. 1, 27 (2018). DOI:10.1038/s42004-018-0027-x
[19] Lin, Z., Kabe, R.*, Nishimura, N., Jinnai, K., Adachi, C.*
Organic long-persistent luminescence from a flexible and transparent doped polymer
Adv. Mater. 30, 1803713 (2018). DOI:10.1002/adma.201803713
[Frontispiece]
[18] Jinnai, K., Kabe, R.*, Adachi, C.*
Wide-range tuning and enhancement of organic long-persistent luminescence using emitter dopants
Adv. Mater. 30, 1800365 (2018). DOI:10.1002/adma.201800365
[Frontispiece]
[17] Jinnai, K., Kabe, R.*, Adachi, C.*
A near-infrared organic light-emitting diode based on an Yb(III) complex synthesized by vacuum co-deposition
Chem. Commun. 53, 5457–5460 (2017). DOI:10.1039/C7CC01580A
[16] Kabe, R.*, Adachi, C.*
Organic long persistent luminescence
Nature 550, 384–387 (2017). DOI:10.1038/nature24010
[15] Notsuka, N., Kabe, R.*, Goushi, K., Adachi, C.*
Confinement of long-lived triplet excitons in organic semiconducting host-guest systems
Adv. Funct. Mater. 27, 1703902 (2017). DOI:10.1002/adfm.201703902
[Back Cover]
[14] Noda, H., Kabe, R., Adachi, C.*
Blue thermally activated delayed fluorescence molecule having acridane and cyanobenzene units
Chem. Lett. 45, 1463–1466 (2016). DOI:10.1246/cl.160814
[13] Yanai, N.*, Kozue, M., Amemori, S., Kabe, R., Adachi, C., Kimizuka, N.*
Increased vis-to-UV upconversion performance by energy level matching between a TADF donor and high triplet energy acceptors
J. Mater. Chem. C. 4, 6447–6451 (2016). DOI:10.1039/C6TC01816E
[Front Cover]
[12] Mieno, H., Kabe, R.*, Notsuka, N., Allendorf, M.D., Adachi, C.*
Long-lived room-temperature phosphorescence of coronene in zeolitic imidazolate framework ZIF-8
Adv. Opt. Mater. 4, 1015–1021 (2016). DOI:10.1002/adom.201600103
[11] Kabe, R., Notsuka, N., Yoshida, K., Adachi, C.*
Afterglow organic light-emitting diode
Adv. Mater. 28, 655–660 (2016). DOI:10.1002/adma.201504321
[10] Nguyen, N.T., Mori, Y., Matsumoto, T., Yatabe, T., Kabe, R., Nakai, H., Yoon, K.-S., Ogo, S.*
A [NiFe]hydrogenase model that catalyses the release of hydrogen from formic acid
Chem. Commun. 50, 13385–13387 (2014). DOI:10.1039/C4CC05911E
[09] Kabe, R., Feng, X.*, Adachi, C.*, Müllen, K.*
Exfoliation of graphite into graphene in polar solvents mediated by amphiphilic hexa-peri-hexabenzocoronene
Chem. - An Asian J. 9, 3125–3129 (2014). DOI:10.1002/asia.201402535
[08] Lee, J.-H., Kim, H.-M., Kim, K.-B., Kabe, R., Anzenbacher, P., Kim, J.-J.*
Homogeneous dispersion of organic p-dopants in an organic semiconductor as an origin of high charge generation efficiency
Appl. Phys. Lett. 98, 173303 (2011). DOI:10.1063/1.3569144
[07] Matsumoto, T., Kabe, R., Nonaka, K., Ando, T., Yoon, K.-S., Nakai, H., Ogo, S.*
Model study of CO inhibition of [NiFe]hydrogenase
Inorg. Chem. 50, 8902–8906 (2011). DOI:10.1021/ic200965t
[06] Kabe, R., Lynch, V.M., Anzenbacher Jr., P.*
Enhanced phosphorescence in dibenzophosphole chalcogenide mixed crystal
CrystEngComm. 13, 5423 (2011). DOI:10.1039/c1ce05388d
[05] Kabe, R., Nakanotani, H., Sakanoue, T., Yahiro, M., Adachi, C.*
Effect of molecular morphology on amplified spontaneous emission of bis-styrylbenzene derivatives
Adv. Mater. 21, 4034–4038 (2009). DOI:10.1002/adma.200803588
[04] Nakanotani, H., Kabe, R., Yahiro, M., Takenobu, T., Iwasa, Y., Adachi, C.*
Blue-light-emitting ambipolar field-effect transistors using an organic single crystal of 1,4-bis(4-methylstyryl)benzene
Appl. Phys. Express. 1, 091801 (2008). DOI:10.1143/APEX.1.091801
[03] Shimazaki, Y.*, Kabe, R., Huth, S., Tani, F., Naruta, Y., Yamauchi, O.*
Formation and characterization of Co(III)−semiquinonate phenoxyl radical species
Inorg. Chem. 46, 6083–6090 (2007). DOI:10.1021/ic700596g
[02] Ogo, S.*, Kabe, R., Uehara, K., Kure, B., Nishimura, T., Menon, S.C., Harada, R., Fukuzumi, S., Higuchi, Y., Ohhara, T., Tamada, T., Kuroki, R.
A Dinuclear Ni(µ-H)Ru Complex Derived from H2
Science 316, 585–587 (2007). DOI:10.1126/science.1138751
[01] Ogo, S.*, Kabe, R., Hayashi, H., Harada, R., Fukuzumi, S.*
Mechanistic investigation of CO2 hydrogenation by Ru(II) and Ir(III) aqua complexes under acidic conditions: two catalytic systems differing in the nature of the rate determining step
Dalton Trans. 4657 (2006). DOI:10.1039/b607993h
[Front Cover]



