FY2020 Annual Report
Neurobiology Research Unit
Professor Jeff Wickens

Abstract
The goal of the Neurobiology Research Unit is to understand the cellular mechanisms and neural circuitry underlying learning and adaptive behavior in the mammalian brain. This collaborative, interdisciplinary program of research is focused on the striatum of the basal ganglia and the neuromodulators, dopamine and acetylcholine, which play a central role in the mechanisms of reinforcement learning. Our main achievements have been: to characterize synaptic plasticity in the striatum and its modulation by dopamine; to measure dopamine signaling during learning and its role in the therapeutic mechanisms of methylphenidate; and, to show a role for cholinergic interneurons of the striatum in flexible behavior. These findings are of broad, general significance for the neuroscience of learning and motivation, and of fundamental importance for clinical understanding of major neuropsychiatric disorders. Our research has the forward goal of developing better treatments for attention-deficit hyperactivity disorder and Parkinson’s disease, which are debilitating neurological disorders of great importance to children and adults.
1. Staff
- Dr Andres Carrasco, Staff Scientist
- Dr. Nobuyoshi Kitamura, Staff Scientist
- Dr. Atsushi Tamura, Postdoctoral Scholar
- Dr Julie Chouinard, Postdoctoral Scholar
- Dr Gideon Sarpong, Postdoctoral Scholar
- Dr. Kiyoto Kurima, Technical Staff
- Ms. Yumiko Akamine, Technical Staff
- Mr. Kavinda Liyanagama, Technical Staff
- Dr. Mayank Aggarwal, Junior Research Fellow
- Dr. Stefan Pommer, Junior Research Fellow
- Dr. Sakurako Watanabe, Junior Research Fellow
- Ms. Yoriko Yamamura, PhD Student
- Ms. Lorena Andreoli, PhD Student
- Ms. Bozena Silic, PhD Student
- Mr. Dvyne Nosaka, PhD Student
- Mr. Mao-Ting Hsu, PhD Student
- Mr. Kang-Yu Chu, PhD Student
- Ms. Yukako Suzuki, Research Unit Administrator
- Ms. Sakiko Takahashi, Research Intern/Tohoku Univ Grad of Medicine. Sep 2020-Mar 2021
2. Collaborations
2.1 Focused ultrasound non-invasive stimulation
- Description: Effects of ultrasound on deep brain structures
- Type of collaboration: Joint research
- Researchers:
- Dr Jorge Moll, Mr Clayton Teixeira, D'or Institute for Research and Education, Brazil.
- Professor Jeff Wickens, Gail Tripp, Emi Furukawa, OIST.
2.2 Human Frontier Science Program
- Description: Spatiotemporal neurochemical dynamics of behavioral flexibility in the striatum
- Type of collaboration: Joint research [2019-2022]
- Researchers:
- Dr Joshua A. Goldberg, Dept. of Medical Neurobiology-IMRIC- The Faculty of Medicine, Jerusalem, Israel.
- Dr Lin Tian, Dept. of Biochemistry and Molecular Medicine. Tian Lab, University of California. Davis/School of Medicine, Davis, USA.
- Professor Jeff Wickens, OIST.
2.3 Dopamine signaling and mechanism of pyschostimulant action
- Type of collaboration: Joint research
- Researchers:
- Professor Brian Hyland, University of Otago, New Zealand.
- Professor Jeff Wickens, OIST.
3. Activities and Findings
Our overarching aim is to investigate the neural mechanisms of the striatum of the basal ganglia, and to understand the contribution of these mechanisms to reinforcement learning and behavioral flexibility. As the main input nucleus of the basal ganglia, the striatum receives sensory, cognitive, and motor information from cortical and thalamic afferents, which synapse directly on the output neurons of the striatum, the spiny projection neurons (SPNs). The GABAergic SPNs receive reward signals from midbrain dopaminergic neurons and contextual information from intrinsic cholinergic interneurons (CINs). The SPNs are the principal output neurons of the striatum, and additionally form a lateral inhibitory network with other SPNs via their local axon collaterals. Multiple subtypes of GABA interneurons complete this circuitry. The input-output information processing operation performed by the striatum is not fully understood, but we speculate that this anatomy provides a matrix of modifiable input-output connections (Fig. 1) which compete for control of output, forming a substrate for stimulus-response learning in the dorsolateral striatum, goal-directed learning in the dorsomedial striatum, and reward-related learning in the ventral striatum.
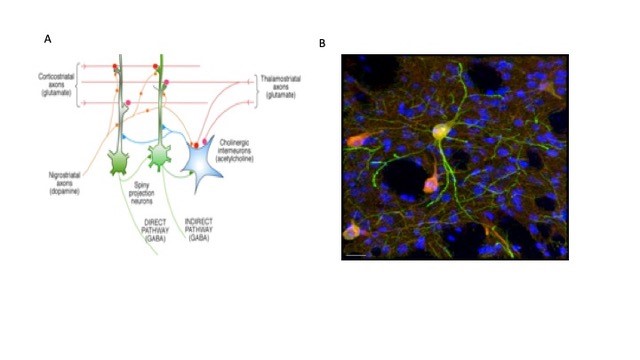
Figure 1. Focus of unit research. A. Convergence of corticostriatal and thalamostriatal afferents on spiny neurons forms matrix of alternative connections, modulated by DA and ACh. CINs control groups of SPNs. B. Doubly labelled CINs shown in merged image of GFP (green) and ChAT (red) in dorsolateral striatum; Hoescht DNA labelling (blue) shows nuclei of all cells, predominantly SPNs. Note the fine red axonal arborization of the CINs envelopes many SPNs (unpublished preliminary data, Julie Chouinard, Andres Carrasco). Scale bar 20 µm.
Effect of serotonin 5-HT1B receptors on lateral inhibition in the striatum
(Stefan Pommer)
Experimental study of the behavioral function of lateral inhibition in the striatum is technically impossible at present because there is no way to turn off lateral inhibition by SPNs without also compromising either the effect of SPNs on projection areas (if the SPNs are targeted) or the function of the local interneurons (if GABA is targeted). One approach is to identify receptors that may be selectively expressed on axon collaterals of SPNs, as a possible target. Serotonin, acting via the 5-HT1B receptor, modulates neurotransmitter release from SPN terminals in striatal output nuclei, but the role of 5-HT1B receptors in lateral inhibition among SPNs in the striatum is unknown. We investigated the effects of 5-HT1B receptor activation on lateral inhibition in the striatum.
To measure lateral inhibition we used rAAV5/EF1a-DIO-hChR2(H134R)-eYFP (UNC Vector Core) to express Channelrhodopsin2 in D1 SPNs using Tg(Drd1-Cre)FK150Gsat/Mmucd (Gensat) transgenic mice or presumed D2 SPNs using Adenosine A2A receptor-Cre BAC transgenic mice (Dr. Alban de Kerchove d’Exaerde). The transgenic mice were backcrossed to C57BL/6J more than 12 generations, prior to use. Whole-cell recordings were made from SPNs in acute brain slices, while optogenetically activating presynaptic SPNs to produce inhibitory postsynaptic currents (IPSCs) mediated by release of GABA from SPN collaterals. We measured the effects of 5-HT1B receptor on these IPSCs (Fig. 1).
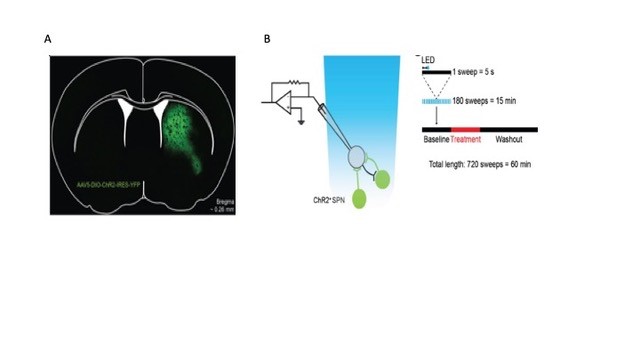
Figure 2: Optogenetic approach to measurement of lateral inhibition. Left. Specific expression of ChR2 in a D1-Cre mouse. Right. Recording protocols for ChR2-evoked IPSCs in voltage clamp configuration. Membrane current is recorded in repeated 5-second sweeps. A single 2 ms light pulse (blue bar) is given one second (double arrowheads) after the start of each sweep. Data from Pommer et al., (submitted) and Pommer (2020)
We found that activation of 5-HT1B receptors significantly reduced the amplitude of averaged IPSCs evoked by optical stimulation of both direct and indirect pathway SPNs (Fig. 2). This reduction was blocked by application of a 5-HT1B antagonist. These results suggest a new role for serotonin as a modulator of lateral inhibition among striatal neurons. The 5-HT1B receptor may, therefore, be a suitable target for future behavioral experiments investigating the currently unknown role of lateral inhibition in the function of the striatum. A paper from this has been submitted and is currently in revision.We are currently extending this project by investigating the modulatory effect of 5HT1B receptors on inhibition mediated by interneurons, and more detailed analysis of the IPSCs using statistical models.
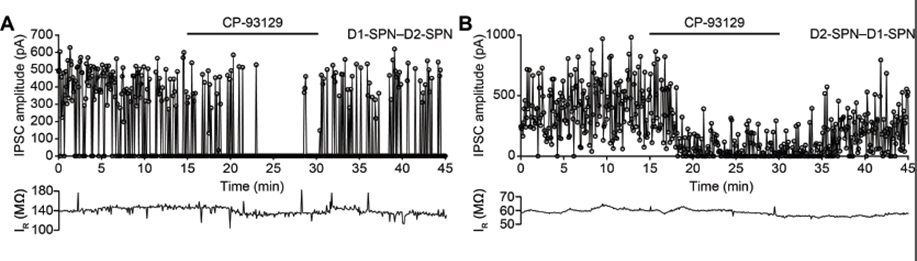
Figure 3: Effect of 5HT1b receptor on lateral inhibition. A IPSC amplitude recorded from D1–D2-SPN connection (D1-Cre mouse) shows effect of bath application of serotonin 5-HT1B receptor agonist CP-93129. B. Same for D2-D1 connection (A2a-Cre mouse). Data from Pommer et al., (submitted) and Pommer (2020).
Spatiotemporal neurochemical dynamics of DA and ACh in the striatum
(Collaboration with Joshua Goldberg, Univ Jerusalem; Lin Tian, UC Davis)
(Julie Chouinard, Kiyoto Kurima)
Genetically encoded indicators for DA and ACh, in combination with transgenic animals and modern microscopy, are new tools for direct, specific and long-term imaging of neuromodulatory signals in target circuits. Lin Tian recently developed an intensity-based genetically encoded indicator for DA – dLight1 – which works by directly coupling the conformational changes of an inert human DA receptor to changes in the fluorescence intensity of a circularly permuted green fluorescent protein (cpGFP). This new technology provides neurochemical sensing with ultra-fast temporal resolution while also providing cellular and subcellular resolution and high molecular specificity. These properties of dLight1 permit robust and long-term detection of behaviorally relevant DA transients. For example, in freely moving mice, dLight1 permits deep-brain recording of bi-directional DA dynamics
In 2019 we established a collaboration with Lin Tian (UC Davis) and Josh Goldberg (Jerusalem) to study spatiotemporal dynamics of DA and acetylcholine in the striatum. We obtained an HFSPO grant which provided funding for some of the collaborative elements of the program, starting December 2019. In the first year of the project we have established working relationships with Josh Goldberg and Lin Tian. The Tian group is providing these plasmids for sensors, which we then package with adeno-associated virus to be driven by various promoter and serotypes. We are testing the new constructs in brain slice preparations, applying them in vivo, and sharing data and viruses with the Goldberg group who are testing them using endoscopic imaging in free-moving animals. We have produced AAV5-hSyn-dLight1.3b and AAV5-CAG-Flex-dLight1.3b viruses for the neuronal and Cre-dependent versions respectively.
At OIST we have completed the assembly and testing of a custom two-photon microscope designed in consultation with INSS, and configured it with a virtual reality system for in vivo imaging where mice can move on an air-cushioned spherical treadmill surrounded by a monitor array controlled by Phenosys software. An ultrafast tuneable pulsed laser with wide tuning range provides long wavelength capabilities for future developments in red-shifted sensors and a resonant xy scanner provides high frame rates for dynamic in vivo imaging. Two independently scanned light paths and laser sources provide for imaging and optogenetic stimulation. The microscope is controlled by ScanImage software, open source software that provides flexible control of the microscope. A head-restraint has been configured within the virtual reality space. This configuration provides the mechanical stability needed for the highest resolution measurements using two-photon imaging to observe fine structure.
In parallel with the construction of the 2-photon microscope, in preparation for our awake animal experiments, we have been validating dLight1.3b, and the ACh sensor iAChSnFR, using brain slices, confocal and 2 photon microscopy, and epifluorescence (Fig 4). In evaluations of dLight variants we have used simultaneous recording of optical signals and fast-scan cyclic voltammetry (FSCV) measurements.
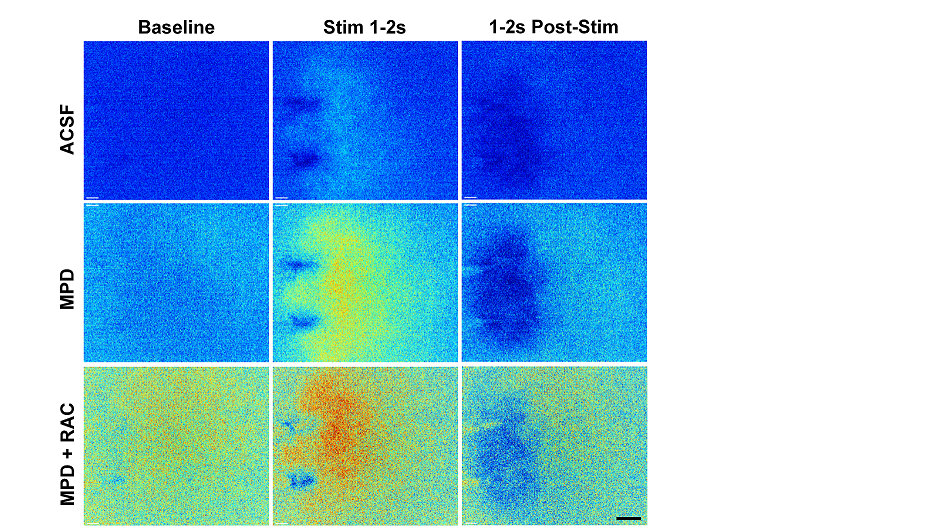
Figure 4: Preliminary studies using dLight1.3b under control (ACSF), methylphenidate 30 mM (MPD) and MPD plus 1 mM Raclopride (MPD+RAC) conditions. Each F/F0 image is the average of all frames acquired during 1 second (Baseline, 10 frames) before electrical field stimulation using a bipolar electrode, visible in images. Stim 1-2 s, last second of stimulation. 1-2s Post-Stim, response 1s after end of stimulation. Rainbow scale ranges between lowest (dark blue) and highest (red) fluorescence values. Stimulation 20 pulses, 2ms/phase, at 10Hz. Scale bar 200 µm. Paper in preparation, Julie Chouinard.
These have revealed differences between the two measures that may be related to the different compartmental expression of dLight. Depending on how it is expressed, it may sample from the presynaptic perisynaptic space or the postsynaptic cell membrane, in contrast to the FSCV carbon fiber electrode, which samples the extracellular space and is influenced by physicochemical interactions such as adsorption of DA onto the electrode surface. For example, the dLight signal timecourse does not show the classical “overshoot” that is seen with FSCV in methylphenidate, in which the peak concentration occurs some time after the end of the stimulation, and the clearance is orders of magnitude slower. These experiments have helped us to understand what dLight is reporting and also helped define the optimal filtering and excitation parameters to obtain the best signal to noise ratios and least photobleaching. The 2D view that dLight provides suggests that methylphenidate has effects on apparent diffusion distance of DA, which may be important for understanding its effects on processing of reward signals and its mechanisms of action in the treatment of attention-deficit hyperactivity disorder.We will soon be evaluating dLight2.1 which is brighter and more sensitive than the dLight1 variants, and also evaluating red dLight variants for use in two-channel recording.
Sensory processing and cholinergic interneuron properties in transgenic mice
(Andres Carasco, Atsushi Tamura, Julie Chouinard, Stefan Pommer, Kiyoto Kurima )
Advances in gene manipulation techniques have provided mouse lines that express Cre-recombinase under the choline acetyltransferase (ChAT) promoter. These transgenic strains permit the targeted introduction of opsins in cholinergic neurons by gene recombination or adeno-associated viral injection. However, experience teaches that transgenic animals may differ from the wild type in unintended ways. In order to increase confidence about the use of these manipulations on CIN function and sensory processing we investigated cellular properties of CINs and auditory processing in ChAT-Cre and ChAT-ChR2(Ai32) mice. Figure 5 shows images from a ChAT-ChR2(Ai32) mouse, confirming that the YFP positive cells are also ChAT positive, and showing the many SPNs nuclei (blue) surrounding each CIN.
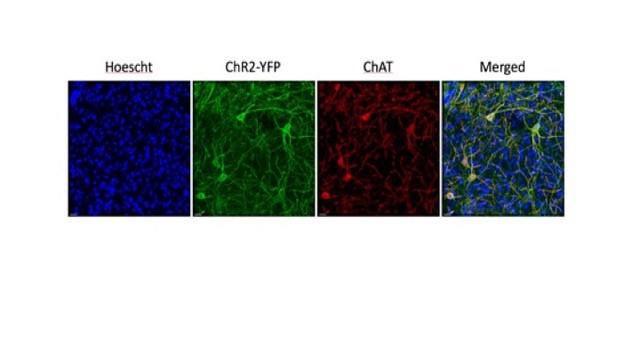
Figure 5: CINs surrounded by SPNs in ChAT-ChR2(Ai32) mouse striatum. Double labelling of CINs (YFP and ChAT immunoreactivity) confirms identity of green or red neurons as cholinergic cells. Blue Hoescht staining shows location of SPNs. Scale bar 20µm. Unpublished data, Julie Chouinard.
We found that these transgenic animal lines hear as well as C57BL/6J mice. To examine possible effects on the electrophysiological properties of striatal CINs, responses to hyperpolarizing and depolarizing current pulses were compared. Whole-cell recordings from single CINs showed no obvious differences between ChAT-Cre and ChAT-ChR2(Ai32) and C57BL/6J mice and statistical analysis showed no significant differences in resting membrane potential, spike amplitude, spike width, input resistance, threshold, after-hyperpolarizations, or spontaneous firing. Significant differences were noted only in sag potential between wild type and transgenic mice which might suggest the presence of variations in hyperpolarization-activated cation currents (iH) between animal strains. Further work will be required to validate and characterize plausible variations in iH of opsin-infected CINs. However, overall these findings support the use of these mouse lines in behavioral experiments involving auditory cues.
4. Publications
4.1 Journals
- Aggarwal, M. and Wickens, J.R. (2021) Reward influences cortical representations. Commentary on the paper "The growth of cognition: free energy minimization and the embryogenesis of cortical computation" by Wright and Bourke. Physics of Life Reviews 36, 3-4. https://pubmed.ncbi.nlm.nih.gov/33278814/
- Aggarwal, M. and Wickens, J.R. (2021) Behavioral determinants in the expression of the Kamin blocking effect: Implications for associative learning theory. Neuroscience & Biobehavioral Reviews,124:16-34. https://pubmed.ncbi.nlm.nih.gov/33497781/
- Aggarwal, M. and Wickens, J.R. (2020) The Kamin blocking effect in sign and goal trackers. bioRxiv doi: https://doi.org/10.1101/2020.08.01.232553. https://pubmed.ncbi.nlm.nih.gov/33497781/
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Akamine, Y. Oral Presentation, Title of talk(oral in Japanese): "Ticket to Science". HiSci-Lab, March 13, 2021. 恩納村ふれあい体験学習センター, Onnason, Okinawa.
「ハイサイ・ラボでは、これまでの琉球ガールズの活動内容を引き継ぎ、 理系分野への女性の進出を後押しする為、県内女子高校生の理系分野への進路選択を支援したイベントです。」
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 PhD Thesis Presentation by Ms. Sakurako Watanabe
Title of Talk: "Interaction of multiple inputs in plasticity of the corticostriatal synapses"
Date: Monday, November 30, 2020. 13:00-14:00.
Venue: Zoom
Speaker: Ms. Sakurako Watanabe, Neurobiology Research Unit, OIST



