FY2020 Annual Report
Molecular Neuroscience Unit
Assistant Professor Marco Terenzio

(From left to right) Lower row: Yoko Nakano, Sandra De La Fuente, Madeleine Le Coz, Lokesh Agrawal. Upper row: Maria Fransiska Emily, Sara Emad El-Agamy Abdelaal, Laurent Guillaud, Marco Terenzio and Maria Fernanda Bolanos Alejos.
Abstract
From the 1rstof April 2020 we had access to our laboratory space in Laboratory 4. We have fitted and organized the laboratory space and purchased all the necessary equipments. We have recruited 3 postdocs, 1 Ph. D. student and 1 technician (see list below). During FY2020 we have published one review paper and submitted a method paper in Methods in Molecular Biology. This paper is about the development of microfluidics chambers to culture and image adult sensory neurons and the primary contributor his Maria Emily, a Ph. D. student of the unit. We have also developed several research areas, generated a transgenic mouse models and established a protocol for differentiation of hIPSC in motor neurons.
1. Staff
- Assistant Professor Marco Terenzio, Unit Leader
- Dr. Laurent Guillaud, Group Leader
- Dr. Lokesh Agrawal, Post-doctoral Scholar
- Dr. Madeleine Le Coz, Post-doctoral Scholar
- Dr. Sandra De La Fuente, Post-doctoral Scholar
- Sara Emad El-Agamy Abdelaal, Ph.D. Student
- Maria Fransiska Emily, Ph.D. Student
- Yoko Nakano, Laboratory Technician
- Maria Fernanda Bolanos Alejos, Rotation Student (January 2021-)
- Lilian Magnus, Rotation Student (January 2021-)
- Akiko Guzman, Research Unit Administrator
2. Collaborations
2.1 Local Translation and axonal dysfunction in Amyotrophic Lateral Sclerosis
- Type of collaboration: Joint research
- Researcher:
- Professor Andrea Malaspina, Queen Mary University London, UK
2.2 Local axonal and synaptic maintenance mechanisms in a Drosophilamodel of Wallerian degeneration
- Type of collaboration: Joint research
- Researchers:
- Assistant Professor Lukas Neukomm, University of Lausanne, Switzerland
- Maria Paglione, Ph.D. student
3. Activities and Findings
3.1 The role of Dynein-dependent retrograde axonal transport in sensory and motor neurons
Neurons are highly polarized cells with an elongated axon that extends far away from the cell body. In order to maintain neuronal homeostasis, neurons rely extensively on axonal transport of membranous organelles and other molecular complexes. Axonal transport plays a central role in the establishment of neuronal polarity, axonal growth and stabilization and synapses formation, allowing for precise spatio-temporal activation and modulation of numerous molecular cascades. Anterograde and retrograde axonal transport is supported by various molecular motors, such as kinesins and dyneins, and a complex microtubule network. Cytoplasmic dynein is the main retrograde molecular motor and is constructed around the heavy chain, which is the force-generating subunit and is composed of a motor and a tail domain. The former is responsible for motility generation while the latter acts as a platform for the association of other subunits, such as the intermediate, light-intermediate and light chains, which collectively mediate the direct and indirect binding/association of cargos. Roadblock 1 (DYNLRB1) is one of the 3 light chain families and was presumed to be an accessory subunit for specific cargoes. Recent work demonstrated that DYNLRB1 depletion in proprioceptive neurons significantly impairs retrograde axonal transport and consequently compromises neuronal survival (Terenzio et al., 2020). This observation suggests that DYNLRB1 is an essential subunit for dynein-mediated transport.
We have started to perform experiments aimed at the investigation of the molecular mechanisms behind the role of DYNLRB1 in axonal retrograde transport. We have noticed that DYNLRB1 is incorporated only in a subset of Dynein complexes. Therefore, we decided to characterize DYNLRB1 interactors. To this extent we created several fusion constructs of DYNLRB1 and a promiscuous biotinylating enzyme to selectively label DYNLRB1’s interactors and identify them by mass spectrometry. We are infecting DRG neurons with our fusion constructs (Figure 1), we will perform a proteomic screen and proceed characterizing the most promising candidates in vitroand in vivoand their potential implication in the genesis of neurological disorders.
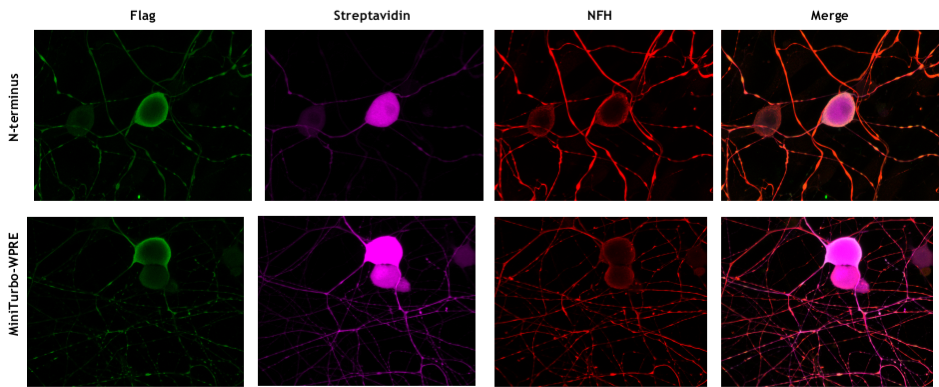
Figure 1: DRG neurons transduced withDYNLRB1 constructs. Successful transduction of our constructs was confirmed by the following stainings. DYNLRB1-miniturbo and miniturbo enzyme are tagged with Flag (in green). Biotinylated proteins are revealed with Streptavidin (in magenta). Neurons are stained with Neurofilament Heavy (NFH, in red).
3.2 Local Translation and axonal dysfunction in Amyotrophic Lateral Sclerosis
The project is about understanding what drives the rate of progression of neurodegeneration, looking at a paradigm of fatal neurodegenerative disorders: Amyotrophic Lateral Sclerosis (ALS). To overcome the limitations of murine models, which recapitulate the most salient features of the human disease, but are not a complete surrogate of the human pathology, we directly derive motor neurons (MNs) from ALS patient’s fibroblasts to assess how local mechanisms of protein production (translation) is changed in motor cells from ALS patients. We established a differentiation protocol for human pluripotent stem cells (iPSC) into MNs using commercially available hiPSC lines (Figure 2A). Successful generation of MNs has been confirmed by immunofluorescence analysis withestablished MN markers such as ChAT and the transcription factor HB9 (Figure 2B&C).
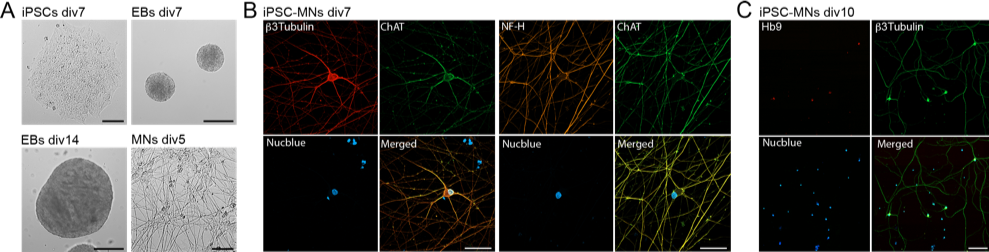
Figure 2: Chemically induced differentiation of human iPSCs into motor neurons.A) Chemical induction of iPSCs and formation of embryoid bodies (EBs) to obtain neural progenitor cells and differentiation into motor neurons (MNs). Representative image of iPSCs colony at div7 (bar = 30 mm), EBs at div7 in the presence of SB and LDN (neuralization and caudalization, bar = 200 mm) and div14 in the presence of SAG and PurM (ventralization, bar = 200 mm), and MNs dissociated culture after 5 days in the presence of CNTF and GDNF (bar = 100 mm). B) Immunofluorescence images showing MNs after 7 days in culture, fixed and labeled with neuronal markers b3Tubulin (left panel) or NF-H (right panel) and ChAT (bar = 40 mm). C) Immunofluorescence images showing MNs after 10 days in culture, fixed and labeled with motor neuron specific marker Hb9 and b3Tubulin (bar = 50 mm).
4. Publications
4.1 Journals
- Anterograde Axonal Transport in Neuronal Homeostasis and Disease. L Guillaud, SE El-Agamy, M Otsuki, M Terenzio. Frontiers in Molecular Neuroscience 13, 179 (2020)
- A Ca2+-Dependent Switch Activates Axonal Casein Kinase 2α Translation and Drives G3BP1 Granule Disassembly for Axon Regeneration. P.K. Sahoo, A.N. Kar, N. Samra, M. Terenzio, P. Patel, S. J. Lee, S. Miller, E. Thames, B. Jones, R. Kawaguchi, G. Coppola, M. Fainzilber, J. L. Twiss 5. October 15, 2020. Current Biology 0 (2020).
- Importin α3 regulates chronic pain pathways in peripheral sensory neurons. Marvaldi L, Panayotis N, Alber S, Dagan SY, Okladnikov N, Koppel I, Di Pizio A, Song DA, Tzur Y, Terenzio M, Rishal I, Gordon D, Rother F, Hartmann E, Bader M, Fainzilber M. Science. 2020 Aug 14;369(6505):842-846.
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
Nothing to report
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 An importin mechanism in chronic pain
- Date: August 17, 2020.
- Venue: OIST Campus, Seminar Room Lab4 F01 and via Zoom.
- Speaker: Dr. Letizia Marvaldi, Department of Biomolecular Sciences, Weizmann Institute of Science, Israel.
7. Other
Nothing to report.



