FY2021 Annual Report
G0 Cell Unit
Professor Mitsuhiro Yanagida

Abstract
The G0 Cell Unit studies how cells in the proliferative (dividing) or quiescent (non-dividing) phase respond to nutritional shifts (e.g., nitrogen source starvation and glucose limitation). We identify gene products (mostly proteins) and chemical factors (small molecules, metabolites) that affect adaptation mechanisms in order to understand the basis for longevity of long-term quiescent cells. We employ fission yeast as a eukaryotic cell model as the great majority of this organism’s genes are conserved in human. In addition, we are studying chromosomal regulatory mechanisms involving condensin, cohesin complexes (which lead to proper chromosome segregation in proliferating cells), and other nutrient adaptation-related nuclear chromatin proteins. This line of studies aims to understand dynamics of nuclear chromatin in response to nutritional cues. Study on human blood metabolomics initiated from comparative study with fission yeast metabolomics now directly aims to identify and understand the roles of metabolites intimately related to human aging.
Our current principal research projects may be summarized as below:
(1) Metabolomic approach to human aging and aging-related diseases.
(2) Understanding cell regulation in response to nitrogen deprivation.
(3) Understanding cell regulation in response to glucose starvation.
(4) Understanding chromosomal regulatory mechanisms involving condensin, cohesin complexes, and other nutrient adaptation-related nuclear proteins.
In FY2021, we published 6 original articles on research topics including human metabolomic analysis and chromosome regulatory mechanisms in fission yeast and filed 1 patent, as listed in 3. Activities and Findings, 4. Publications, and 5. Intellectual Property Rights and Other Specific Achievements.
1. Staff
- Dr. Xingya Xu, Staff Scientist
- Dr. Michiko Suma, Postdoctoral Scholar
- Ms. Orie Arakawa, Technician
- Ms. Ayaka Mori, Technician
- Ms. Yuria Tahara, Technician
- Dr. Takayuki Teruya, Technician
- Ms. Risa Uehara, Technician
- Ms. Li Wang, Technician
- Ms. Chikako Sugiyama, Research Unit Administrator
2. Collaborations
- Theme: Analysis of human blood metabolites, involved in aging and aging-related diseases
- Type of collaboration: Joint research
- Researchers:
- Dr. Hiroshi Kondoh, Department of Geriatric Medicine, Graduate School of Medicine, Kyoto University
- Dr. Takumi Mikawa, Department of Geriatric Medicine, Graduate School of Medicine, Kyoto University
- Dr. Masahiro Kameda, Department of Geriatric Medicine, Graduate School of Medicine, Kyoto University
- Theme: Comparative analysis of blood metabolome between healthy people and metabolic abnormality patients
- Type of collaboration: Joint research
- Researchers:
- Professor Hiroaki Masuzaki, Division of Endocrinology, Diabetes and Metabolism, Hematology, Rheumatology (Second Department of Internal Medicine), Graduate School of Medicine, University of the Ryukyus
- Theme: Blood metabolome analysis in abnormal cerebral function
- Type of collaboration: Joint research
- Researchers:
- Dr. Yasuhide Fukuji, Director, National Hospital Organization Ryukyu Hospital
- Dr. Naoya Kida, National Hospital Organization Ryukyu Hospital
- Theme: Assessment of the effect of ingestion of health-related functional supplements on human blood metabolite
- Type of collaboration: Joint research
- Researchers:
- Dr. Tomohiro Rogi, Senior Manager, Suntory Wellness Limited
- Dr. Norifumi Tateishi, General Manager, Suntory Wellness Limited
- Dr. Daisuke Takemoto, Deputy General Manager, Suntory Wellness Limited
3. Activities and Findings
3.1 Whole blood metabolomics of dementia patients reveal classes of disease-linked metabolites (From OIST news center article "Signs of dementia are written in the blood, reveals new study" by Dani Ellenby)
The study revealed that the levels of 33 metabolites differed in patients with dementia, compared to elderly people with no existing health conditions. Their findings, published this week in PNAS, could one day aid diagnosis and treatment of dementia.
“Metabolites are chemical substances produced by vital chemical reactions that occur within cells and tissues,” said first author Dr. Takayuki Teruya, who works in the G0 Cell Unit at the Okinawa Institute of Science and Technology Graduate University (OIST). “Our body normally keeps these levels in balance, but as we age and if we develop diseases like dementia, these levels can fluctuate and change.”
Dementia is not just a single disease, but a general term used to describe a set of symptoms, including a slow but typically irreversible decline in the ability to remember, think, make decisions or perform day-to-day activities. Of all aging-associated diseases, dementia is one of the most serious, not only for the patients and their family but for society as a whole, with an estimated 55 million people living with the disease worldwide.
While scientists know that dementia is caused by damage to nerves, the exact cause of this damage, and methods as to how it can be detected and treated have remained elusive.
In the study, the research team analyzed samples of blood collected from eight patients with dementia, as well as eight healthy elderly people. They also collected samples from eight healthy young people to use as a reference. Unlike most studies analyzing blood metabolites, this research included compounds found within red blood cells.
“Blood cells are difficult to handle because they undergo metabolic changes if left untreated even for a short period of time,” explained Dr. Teruya.
However, the research team recently developed a way to stabilize metabolites in red blood cells, allowing them to examine for the first time the relationship between red blood cell activity and dementia.
The scientists measured the levels of 124 different metabolites in whole blood and found that 33 metabolites, split into 5 different sub-groups, correlated with dementia. Seven of these compounds increased in dementia patients, whilst 26 of these compounds showed a decrease in levels. 20, including nine that were abundant in red blood cells, of these compounds had not previously been linked to dementia.
“Identification of these compounds means that we are one step closer to being able to molecularly diagnose dementia,” said senior author of the study, Professor Mitsuhiro Yanagida, who leads the G0 Cell Unit at OIST.
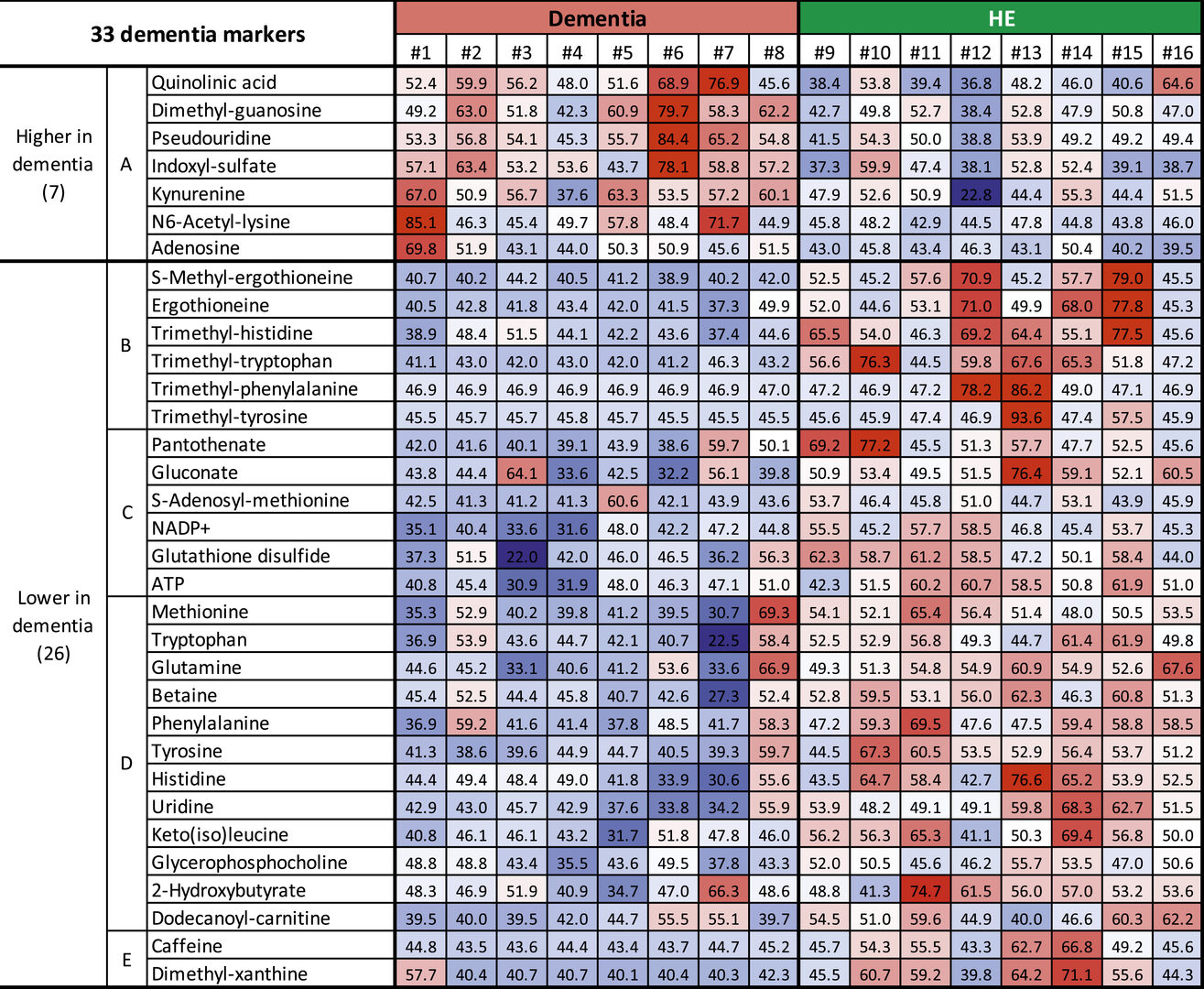
The seven metabolites that showed increased levels in patients with dementia were found within the blood plasma and belonged to sub-group A of metabolites. Importantly, some of these compounds are believed to have toxic effects on the central nervous system.
“It’s still too early to say, but it could suggest a possible mechanistic cause of dementia as these compounds may lead to impairment of the brain,” said Prof. Yanagida.
The research team plans to test this idea in the next steps of their research, by seeing if increases in these metabolites can induce dementia in animal models, like mice.
The remaining 26 compounds that decreased in patients with dementia, compared to healthy elderly people, belonged to four other metabolite sub-groups, B-E.
Six metabolites that decreased in dementia patients were classified into sub-group B, due to their similar structure. These metabolic compounds are antioxidants, which protect cells and tissues by reducing damage caused by free radicals – unstable molecules produced by chemical reactions in cells. The researchers found that these antioxidant compounds derived from food were highly abundant in red blood cells of healthy elderly people.
“It could be that red blood cells deliver not only oxygen but also crucial metabolites that protect the nervous system from damage,” said Dr. Teruya.
The remaining sub-groups contain compounds that the researchers believe play a role in supplying nutrients, maintaining energy reserves and protecting neurons from damage.
“In the future, we hope to start some intervention studies, either by supplementing dementia patients with metabolic compounds in sub-groups B-E, or by inhibiting the neurotoxins from sub-group A, to see if that can slow, prevent, or even reverse symptoms of dementia,” said Prof. Yanagida.
The research was conducted by the Okinawa Institute of Science and Technology Graduate University, along with the National Ryukyu Hospital, Okinawa and Kyoto University.
3.2 Human age-declined saliva metabolic markers determined by LC-MS (From OIST news center article "How we age, as told by spit" by Lucy Dickie)
Researchers in Japan have conducted a comprehensive analysis of the metabolites that make up human saliva using samples given voluntarily from a group of 27-to-33-year-old individuals and a group of 72-to-80-year-old individuals. Metabolites are the intermediate or end products from the chemical reactions that occur within our bodies. They can be related energy synthesis, digestion, growth, cell health, and more. Writing in scientific reports, the researchers from the G0 Cell Unit at the Okinawa Institute of Science and Technology Graduate University (OIST) identified 99 metabolites within the samples of saliva. Notably, the quantities of 21 of these metabolites were different between the two groups.
“Saliva has not previously been comprehensively studied for the changes that occur as one ages,” explained Prof. Mitsuhiro Yanagida who leads OIST’s G0 Cell Unit. The Unit has already looked at blood and urine and found that the quantities of some metabolites are linked to frailty and dementia. Prof. Yanagida explained that having these indicators means that early diagnosis and intervention should be possible. “Frailty and dementia, conditions that are associated with aging, make the day-to-day lives of patients very difficult. Saliva also has a close connection to mouth health. If a mouth does not function correctly, it can make consuming food challenging, which is highly detrimental to one’s quality of life. I hope through this research we can better support elderly people.”
The sample collection was easy and noninvasive. Twenty-seven volunteers in Okinawa supplied their saliva, which they collected themselves at home. These were transferred to the laboratory for analysis. In general, the concentration of metabolites in saliva is very low compared to that in blood and urine, making it more challenging to detect them. However, using a comprehensive method, the researchers identified 99 metabolites, some of which were previously unreported in saliva. They also found that saliva contains information that reflects biological aging. Twenty metabolites, including those related to antioxidative activity, energy synthesis, and muscle maintenance, were lower in the elderly individuals than the young people, whereas one metabolite actually increased.
“It’s interesting that ATP, the metabolite related to energy production, increased 1.96-fold in the elderly,” commented lead author, Dr. Takayuki Teruya. “This is possibly due to reduced ATP consumption in the elderly individuals. Amongst the metabolites that declined in quantity were two that are related to taste, suggesting that the elderly lose some ability to taste, and others that are related to muscle activity such as swallowing. These age-linked salivary metabolites together illuminate a metabolic network that reflects a decline of oral function during human aging.”
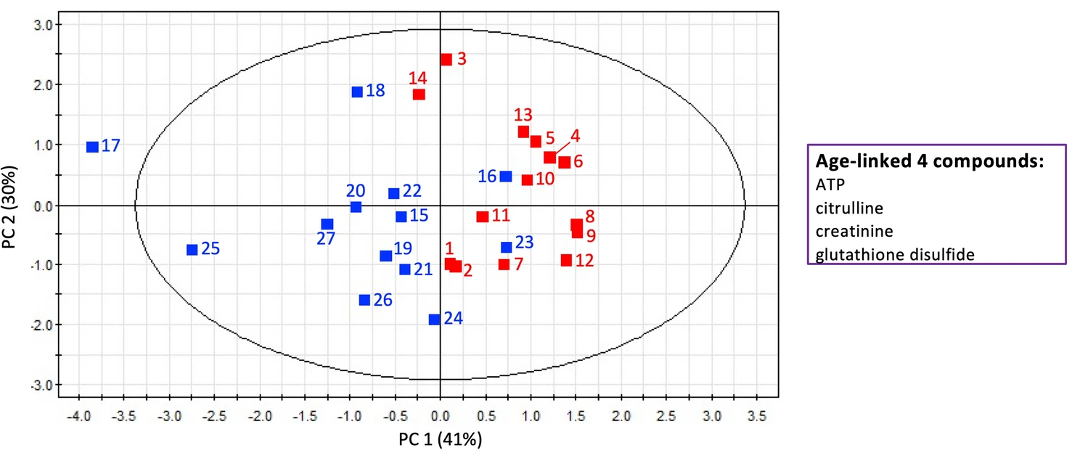
Two metabolites—creatinine and acetyl-carnosine—showed a significant difference between men and women, with women having lower quantities than men. These metabolites are linked to muscle activity.
“In Japan, 90% of people over 90 years of age are women,” said Prof. Yanagida. “And many of them have difficulty with their muscles and cognition. Therefore, when we think about the welfare of the elderly, we must think about women’s health.”
Although this is the first comprehensive analysis to be performed on the metabolites of saliva, the researchers are planning to continue this work. In the future, they hope that saliva will be a sample that can be given readily and easily but could provide an enormous amount of information about an individual’s health.
“In saliva, age-linked metabolites are related to relatively broad metabolic conditions so that age-related information obtained from salivary metabolites may be distinct from that of blood and urine. The results of this study may be useful in the future to assess easily the degree of metabolic aging in humans or to find early indications of age-related diseases.” concluded Prof. Yanagida.
The research was conducted by the G0 Cell Unit, Okinawa Institute of Science and Technology Graduate University, along with Mr. Haruhisa Goga, a trainee from the Department of Criminal Investigation, Okinawa Prefectural Police HQ.
3.3 Reduced uremic metabolites are prominent feature of sarcopenia, distinct from antioxidative markers for frailty
Frailty and sarcopenia are well known as aging-related diseases. Both diseases are increasing with estimated global populations of about 120 million and 90 million individuals, respectively. The clinical evaluation for sarcopenia is different from that for frailty. Sarcopenia is defined as a loss of skeletal muscle and muscle strength in the elderly, while frailty is a state of vulnerability to several stressors, due to declined function or impairment of organs and tissues during aging. Frailty encompasses multiple domains of aging, including cognitive impairment, hypomobility, and decreased social activity.
Although the average ages of both frail and non-frail populations were more than 80 years in our study, 5 of 15 frailty-related metabolites overlapped with aging markers (Chaleckis et al., 2016; Kameda et al., 2020), indicating an intriguing metabolic link between frailty and human aging. Here previous metabolomic data from the study of frailty diagnosed using the EFS were analyzed, based on sarcopenic diagnoses in the same group.
All metabolomic profiles in these participants were analyzed by LC-MS. The 131 metabolites detected were confirmed using standard compounds or by MS/MS, as previously reported. We performed a comprehensive assessment of 131 metabolites based on the diagnosis of sarcopenia. We identified 22 compounds as sarcopenic markers, which did not overlap with 15 frailty markers in the same dataset (Figure 3). Among these 22 compounds, 21 metabolites (acetyl-carnitine, dimethyl-proline, phenylalanine, dimethyl-arginine, N1-methyl-histidine, isovaleryl-carnitine, myo-inositol, creatinine, pantothenate, hypoxanthine, dimethyl-guanosine, N1-methyl-adenosine, 2-oxoglutarate, pentose-phosphate, succinate, N-acetyl-glutamate, quinolinic acid, 4-guanidinobutanoate, N1-methyl-guanosine, trimethyl-tyrosine, and cis-aconitate) decrease significantly in sarcopenia, while aspartate increases. In addition, comparisons between low- and normal-SMI groups identified 10 SMI markers: urate, butyro-betaine, dimethyl-arginine, N1-methyl-histidine, isovaleryl-carnitine, creatinine, hippurate, dimethyl-guanosine, 2-oxoglutarate, and cis-aconitate. All 10 SMI markers are significantly decreased in the low-SMI group. Thirteen of 22 sarcopenia markers and all 10 SMI markers were significantly correlated with SMI. As three SMI markers (urate, butyro-betaine, and hippurate) did not overlap with sarcopenia markers, a total of 25 metabolites are identified as sarcopenia-related markers.
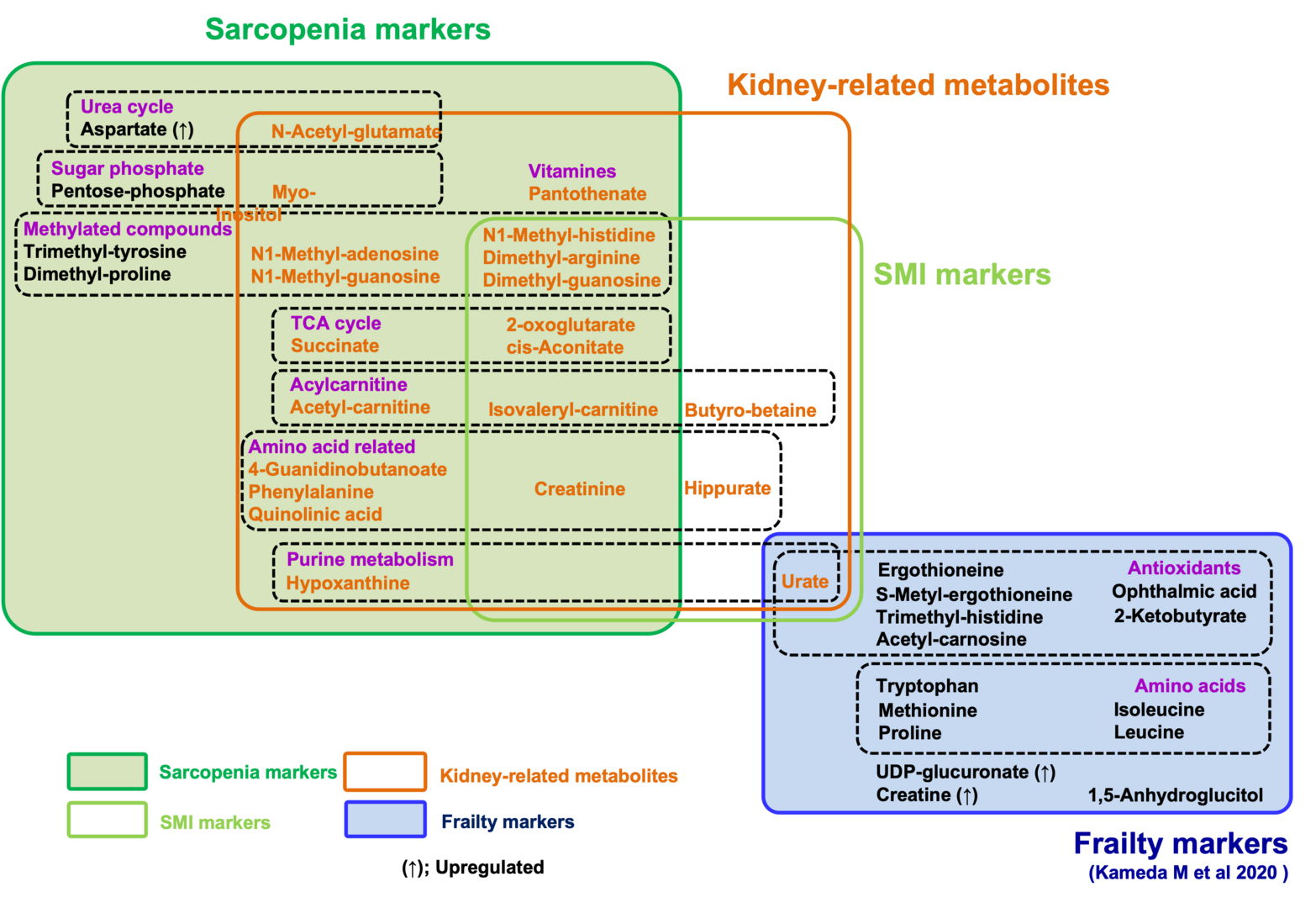
Strikingly, we observed that compounds that decrease in sarcopenia largely overlap with those that increase in kidney diseases. In addition to the list of 10 sarcopenia markers relevant to kidney function, several reports suggest that other sarcopenic metabolites, relevant to mitochondria (9) and methylation (6), are also increased in uremia or kidney disease. Thus, the majority of decreased markers in sarcopenia or low SMI (21 of 24 metabolites) overlap with uremic compounds, which increase in renal dysfunction or uremia. It was also well known that creatinine, a kidney biomarker, declines during muscle loss. Our findings suggest the possibility that waste actively generated via muscle metabolism could be a major burden for kidneys, failure of which results in increased levels of these sarcopenic markers. Alternatively, blood sarcopenia markers might decline by their enhanced excretion from kidney in sarcopenia.
The 22 sarcopenia markers are largely distinct from 15 frailty markers in the same patients, suggesting that metabolic profiles distinguish sarcopenia from frailty. Thus, sarcopenia can be characterized as muscle aging with a decrease of metabolites for mitochondria, muscle, kidney, and methylation, in sharp contrast to the decrease of metabolites for antioxidation in frailty. These findings help not only our understanding of pathogenesis of sarcopenia and frailty, but also future development of clinical applications.
The research was conducted along with Dr. Hiroshi Kondoh and Dr. Masahiro Kameda, Graduate School of Medicine, Kyoto University.
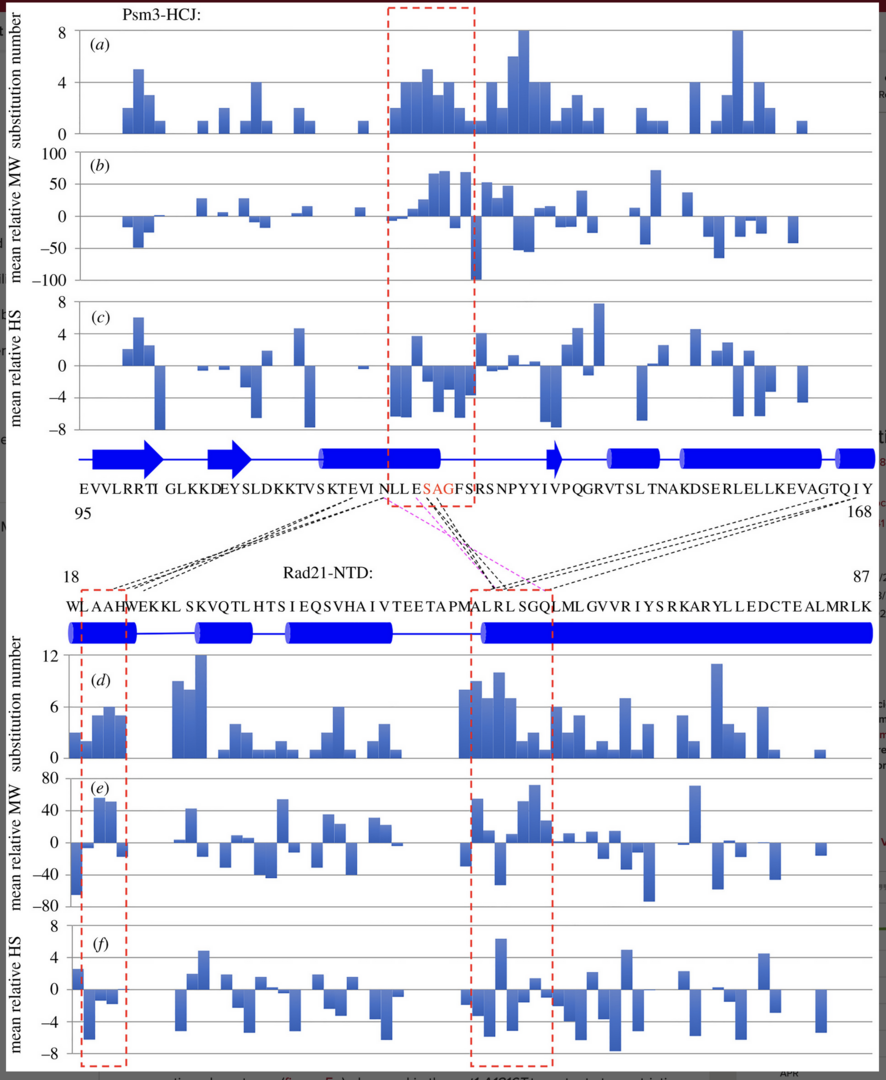
3.4 Single amino acid substitutions in hydrophobic cores at a head-coiled coil junction region of cohesion facilities its release of DNA during anaphase.
Cohesin holds sister chromatids together and is cleaved by separase/Cut1 to release DNA during the transition from mitotic metaphase to anaphase. The cohesin complex consists of heterodimeric structural maintenance of chromosomes (SMC) subunits (Psm1 and Psm3), which possess a head and a hinge, separated by long coiled coils. Non-SMC subunits (Rad21, Psc3 and Mis4) bind to the SMC heads. Kleisin/Rad21’s N-terminal domain (Rad21-NTD) interacts with Psm3’s head-coiled coil junction
(Psm3-HCJ). Spontaneous mutations that rescued the cleavage defects in temperature-sensitive (ts) separase mutants were identified in the interaction interface, but the underlying mechanism is yet to be understood. Here, we identified 113 single amino acid substitutions in Psm3-HCJ and 188 single amino acid substitutions in Rad21-NTD from revertants of the cut1-A1816T ts mutant, respectively (Figure 4).
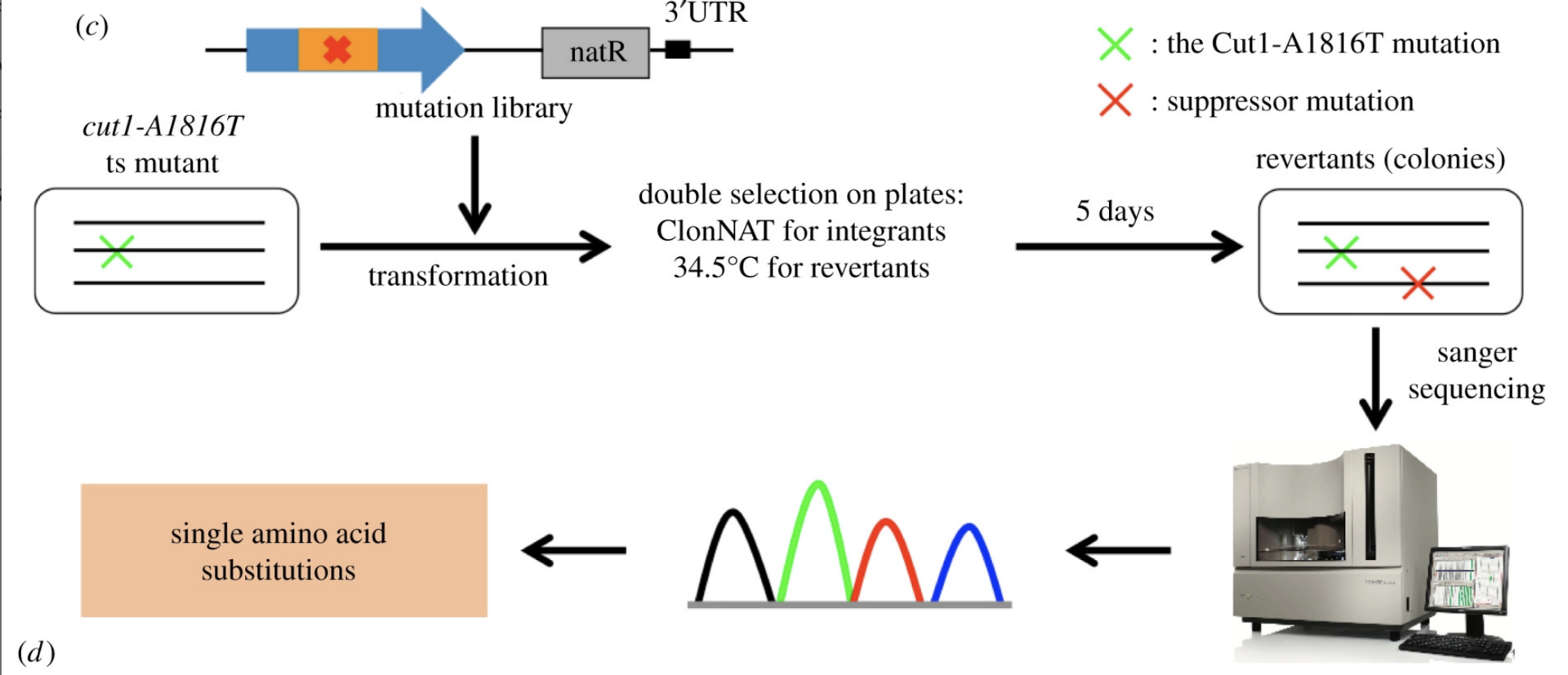
The interaction between Psm3-HCJ and Rad21-NTD may be destabilized by suppressors
Besides the well-known interaction between Rad21-NTD and Psm3’s coiled coil close to its head, Rad21-NTD may also interact with the Psm3 head. We realized that Psm3-HCJ amino acid positions 124–131 and Rad21-NTD amino acids 19–22 and 53–59, which are close to each other are hot spots that are frequently mutated to other amino acids in cut1-A1816T suppressors (Figure 5a, b).
The results indicate that the single amino acid substitutions at positions located near the close contacts between Psm3 head and Rad21-NTD tend to be bulkier and more hydrophilic, thereby possibly changing the structures of the Psm3 head and Rad21-NTD.
Chromosome segregation defects in cut1-A1816T were partially rescued
Spot test results indicated that all these single amino acid substitutions at Psm3-S127, -A128 and -G129 partially rescued the temperature sensitivity of the cut1-A1816T ts mutant at the restrictive temperature, but caused sensitivity to DNA damage (Figure 6).

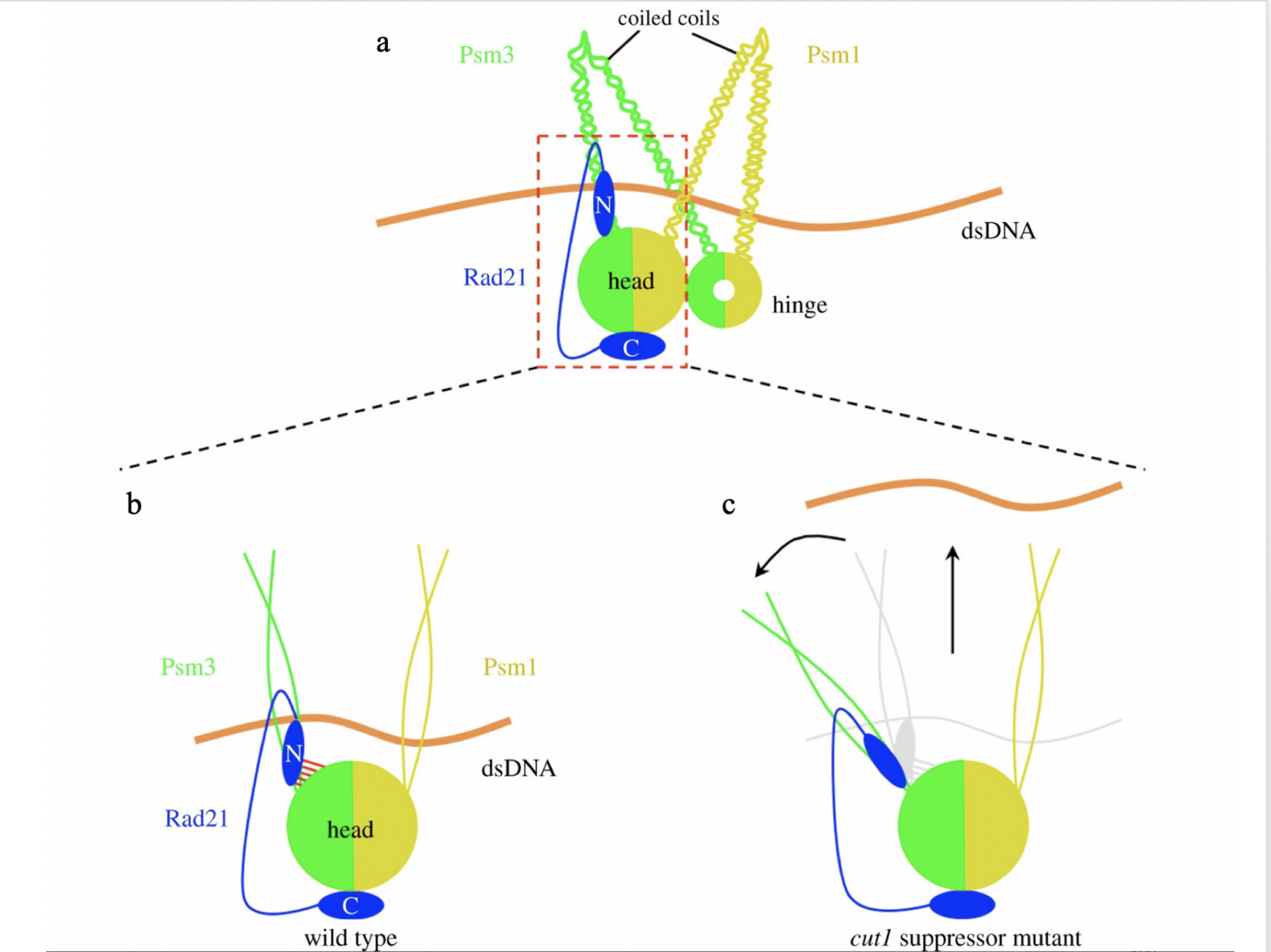
Psm3-HCJ and Rad21-NTD may facilitate sister chromatid separation in a cleavage-independent way through cohesin structural re-arrangement.
This model explains why suppressor mutations, which are supposed to be distant from the separase/Cut1 cleavage sites in Rad21 (R179 and R231) in the three-dimensional structure of the cohesin complex, can rescue cohesin cleavage defects in separase/Cut1 ts mutants and the lethality of uncleavable Rad21. In the wild-type, chromosomal DNA is held tightly by cohesin until its cleavage by activated Cut1/separase.
Suppressor mutations of the cut1-A1816T ts mutant destabilize the interaction between the Psm3 head and Rad21-NTD (Figure 7b, c), which in turn cause cohesin structural rearrangement that may pivot the Psm3 coiled coils away from the Psm1 coiled coils.
4. Publications
4.1 Journals
- Xu X, Kanai R, Wang L, Yanagida M. Single amino acid substitutions in hydrophobic cores at a head-coiled coil junction region of cohesin facilitate its release of DNA during anaphase. Open Biol. 12(4) 2046-2441 [Open Biology]
- Kondoh H, Teruya T, Chen YJ, Fukuji Y, Yanagida M. Reply to Zheng et al.: Clinical metabolomics: detailed analysis by non-targeted method is complementary to large-scale studies. Proc Natl Acad Sci USA (PNAS). 119(5) [PubMed]
- Kondoh H, Teruya T, Kameda M, Yanagida M. Decline of ergothioneine in frailty and cognition impairment. FEBS Letters 10.1002/1873-3468.14299 [PubMed]
- Teruya T, Goga H, Yanagida M. Human age-declined saliva metabolic markers determined by LC-MS. Scientific Reports 11. Article number 18135 [Scientific reports]
- Teruya T, Chen YJ, Kondoh H, Fukuji Y, Yanagida M. Whole blood metabolomics of dementia patients reveal classes of disease-linked metabolites. Proc Natl Acad Sci USA (PNAS). 118(37) [PubMed]
- Kameda M, Teruya T, Yanagida M, Kondoh H. Reduced uremic metabolites are prominent feature of sarcopenia, distinct from antioxidative markers for frailty. Aging (Albany NY) [PubMed]
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Teruya T. Thinking about healthy longevity from changes in metabolism with aging, OIST HiSci Lab 2022, Fureai Taiken Learning Center, Okinawa, 2022.03.26.
- Yanagida M. "Ergothioneine", a dementia marker and functional food, excitement of recent research progress, Annual Meeting of Japan Society for Bioscience, Biotechnology, and Agrochemistry, Online, 2022.03.16.
- Suma M. Fission yeast CENP-C(Cnp3) plays a role in restricting the site of CENP-A(Cnp1) acumulation, National University of Singapore, Online, 2022.03.09.
- Yanagida M. Improvement of understanding of human aging, Fraility, and Dementia (including Alzheimer's disease) and search for treatment methods by comprehensive whole blood metabolome analysis, The 71st Annual Meeting of The Pharmaceutical Society of Japan Kansai Branch, Online, 2021.10.09
- Yanagida M. Study on human metabolites that reflect aging, OIST-Suntory Joint Seminar, Online, 2021.08.27
- Yanagida M. OIST-HUJI Online Workshop, Online, 2021.07.13
- Yanagida M. Living Longer and Healthier: Blue Zones and Aging in the U.S. and JAPAN, OIST Foundation, Online, 2021.04.16
5. Intellectual Property Rights and Other Specific Achievements
Yanagida M, Kondoh H, Teruya T. A method, an apparatus, a system and a kit for determining the extent of aging. Patent granted in Japan, JP2018-548014 (registration date 2021.06.22)
6. Meetings and Events
Nothing to report
7. Other
Nothing to report.



