FY2022 Annual Report
Cellular and Molecular Synaptic Function Unit
Professor Tomoyuki Takahashi
Research Theme: Regulatory mechanisms of transmitter release

Abstract
Synapses play pivotal roles in regulating a variety of brain functions. Such synaptic activities are supported and regulated by synaptic molecules and their interactions. Once homeostatic molecular balance is impaired neurological symptoms such as motor, sensory or cognitive defects can arise depending upon the physiological roles of synapses involved. Thus, it is fundamental to identify the primary molecular/cellular target impaired by pathogenic molecules. As such we have highlighted vesicle endocytosis that is impaired by both α-synuclein inducing Parkinson’s disease (PD) and tau inducing Alzheimer’s disease (AD) in our slice model, where these pathogenic proteins were directly loaded in presynaptic terminals. During this FY, we have developed a culture model in which endogenous pathogenic proteins can be accumulated in presynaptic terminals after chronic treatment with the human pre-formed fibrils of α-synuclein or tau. This model is based on the mechanism of trans-neuronal propagation of pathogenic proteins like prion by which endogenous proteins are seeded by impaired degradation. In this seeding culture model, we have reproduced results in our slice models. Thus, pathogenic protein accumulation causes microtubule over-assembly in presynaptic terminals, thereby sequestering cytosolic dynamins essential for vesicle endocytosis. This culture model also provides a therapeutic platform for screening reagents that prevent pathogenic protein accumulation by stimulating autophagic degradation. So far, we have found one reagent, which reverses α-synuclein seeding. Hence, we have highlighted multiple therapeutic possibilities commonly against PD and AD symptoms, including microtubule disassemblers, microtubule-dynamin binding inhibitor peptide (PHDP5) and an autophagy stimulating reagent. Presently, we are applying these reagents intra-nasally to transgenic tauopathy mice to test whether memory deficits can be rescued in water-maze task.
1. Staff
- Dr. Tetsuya Hori, Group Leader
- Dr. Zacharie Taoufiq, Staff Scientist
- Dr. Satyajit Mahapatra, Staff Scientist
- Dr. Anna Chia-Jung Chang, Postdoctoral Scholar (from April, 2022)
- Dr. Dimitar Dimitrov, Specialist, Technician
- Dr. Marina Khandarkhaeva, Technician
- Dr. Patrick Stoney, Technician
- Ms. Saori Araki, Part-time Research Assistant
- Ms. Asmaa Fawza Yahia Part-time Research Assistant
- Mr. Omar Quaret Sorr, Research Intern (from May to September, 2022)
- Ms. Humaira Noor, Research Intern (from June to September, 2022)
- Ms. Sayori Gordon, Research Unit Administrator
2. Collaborations
2.1 Research theme: Development of cAMP indicator for live neuronal imaging
- Name of partner organization: Doshisha University Faculty of Life and Medical Sciences
- Name of collaboration: Joint research
- Name of principal researcher: Naoto Saitoh
- Name of Researcher: Seiko Kawata
2.2 Research theme: Functional and proteomics analyses of psychiatric diseases at patient’s iPSC-derived synapses
- Name of partner organization: University of the Ryukyus
- Name of collaboration: Scientific Collaboration
- Name of principal researcher: Masayuki Matsushita
- Name of Researcher: Gakuya Takamatsu
2.3 Research theme: Identification of MT-dynamin binding domain
- Name of partner organization: Okayama University
- Name of collaboration: Scientific Collaboration
- Name of principal researcher: Kohji Takei
- Name of Researcher: Hiroshi Yamada
2.4 Presynaptic functional roles of brevican
- Name of partner organization: Kyoto University
- Name of collaboration: Scientific Collaboration
- Name of principal researcher: Takayasu Higo
- Name of Researcher: Takayasu Higo
3. Activities and Findings
3.1 Microtubule assembly by tau impairs endocytosis and neurotransmission via dynamin sequestration in Alzheimer’s disease synapse model (Hori T et al, 2022 eLife 11, e73542).
Recombinant wild-type (WT) monomeric human tau (h-tau) protein infused into the presynaptic terminal reduced EPSCs to ~20 % in 30 min. The rundown of EPSCs by tau was stronger when stimulation frequency was higher.
In capacitance measurements, when tau was loaded by diffusion from presynaptic pipette, endocytic capacitance changes first became slower. Thereafter, exocytic magnitude underwent a decline. These results indicate that the primary target of tau is vesicle endocytosis. As a result of endocytic slowing vesicle recycling is slowed, thereby decreasing exocytosis.
Co-loading of nocodazole (20 mM) with tau into calyceal terminals prevented endocytic impairments by tau and EPSC rundown by tau. Thus, MT disassembler can be a potential therapeutic candidate for symptoms associated with AD as well as with PD.
Vesicle endocytosis highly depends upon the GTPase dynamin and we noticed that dynamin was originally discovered as a MT-binding protein. We then raised a hypothesis that MTs newly assembled by accumulated tau might sequester cytosolic dynamins, thereby blocking endocytosis.
In immunocytochemical analysis tau was loaded from whole-cell pipette and loaded terminals were chemically fixed and permeabilized with a detergent to allow soluble small molecules to be washed out of the terminals to gain signal to noise ratio for bound-form molecular detection. Calyceal terminals loaded with h-tau was identified using h-tau antibody. Tubulin staining in such a terminal revealed an increase in MT. Furthermore, bound fraction of dynamin significantly increased in these terminals. Super-resolution confocal optics analysis of such terminals indicated co-localizations of dynamin with MTs and tau.
Since intra-terminal loading of h-tau induced MT-formation and cytosolic dynamin reduction, interference of MT-dynamin binding could rescue vesicle endocytosis and exocytic transmitter release impaired by h-tau. Since the MT-dynamin binding site is unidentified, we synthesized 24 small peptides corresponding to the different regions of Pleckstrin-homology (PH) domain and proline-rich domain (PRD) of dynamin 1. Among them a dodeca peptide corresponding to 560-571 of PH domain significantly inhibited MT-dynamin 1 binding. This peptide, which we named PHDP5, rescued endocytosis and neurotransmission impaired by tau-loading, when co-loaded into calyceal terminals with tau. Control scramble peptide had no such effect. Thus, PHDP5 might be utilized for AD therapy as well as for PD therapy.
We then conjugated PHDP5 with FITC and amino acid sequence known to permeate the blood-brain-barrier and intra-nasally applied to the transgenic tauopathy mice tau609. We found it entered into olfactory bulbs and then reached the hippocampal region. We are currently testing its learning/memory restoring potency using water maze task.
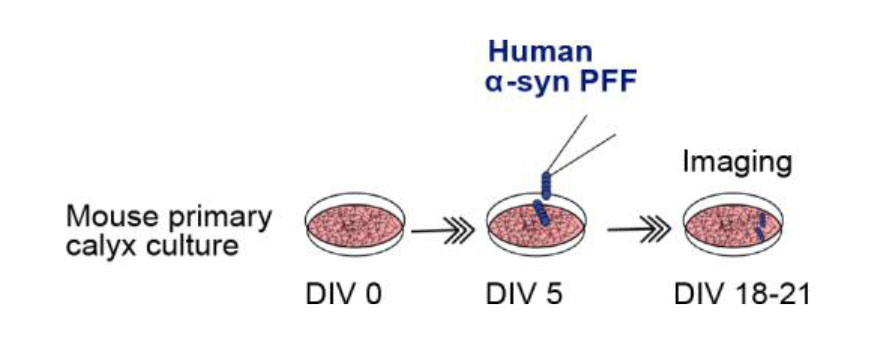
Establishment of culture seeding model for target identification and therapeutic reagent screening for PD and AD (Dimitrov et al, manuscript in preparation).
Chronic treatment of murine cochlear neuronal culture with human α-synuclein preformed fibrils seeded murine endogenous α-synuclein in cochlear neurons, showing accumulation of α-synuclein in calyceal presynaptic terminals.
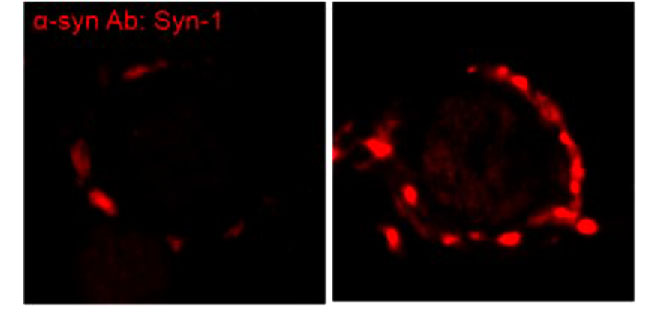
This accumulation of α-synuclein was accompanied by MT binding. Vesicle acidification pHluorin assay indicated impaired endocytosis in such terminals. Likewise chronic treatment of culture neurons with human tau PFF caused accumulation of murine endogenous tau, which over-assembled MT thereby impaired vesicle endocytosis in presynaptic terminals. Lenti-viral infection of dynamin 1 rescued this endocytic impairment by tau. Thus, results obtained in slice model are fully reproduced in this culture model, indicating that accumulation of endogenous pathogenic proteins in presynaptic terminals causes synaptic dysfunction during trans-neuronal propagation. This culture seeding model will provide a platform on which reagents blocking AD/PD progression can be screened.
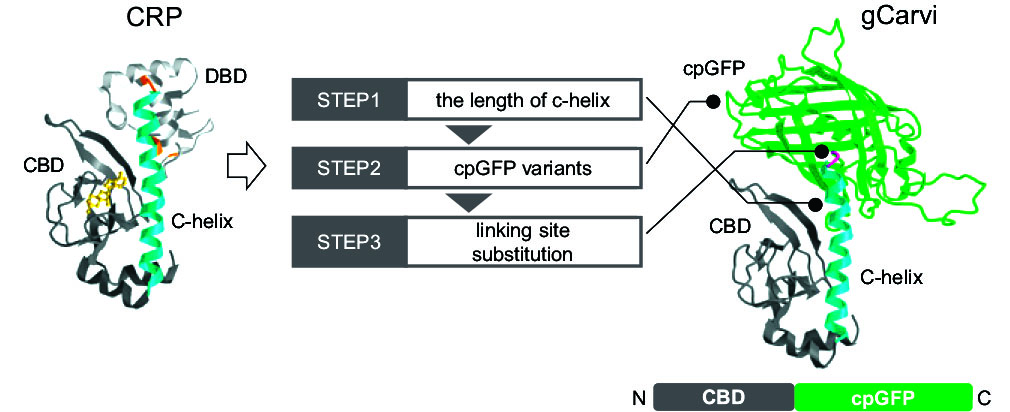
Development of fast and specific cAMP indicator for live neuronal imaging
(Kawata et al, 2022 PNAS 119, e2122618119).
Based upon a bacterial cAMP-binding protein, we have developed a highly specific cAMP indicator covering the range of 0.2-20 mM with a sub-second time resolution.
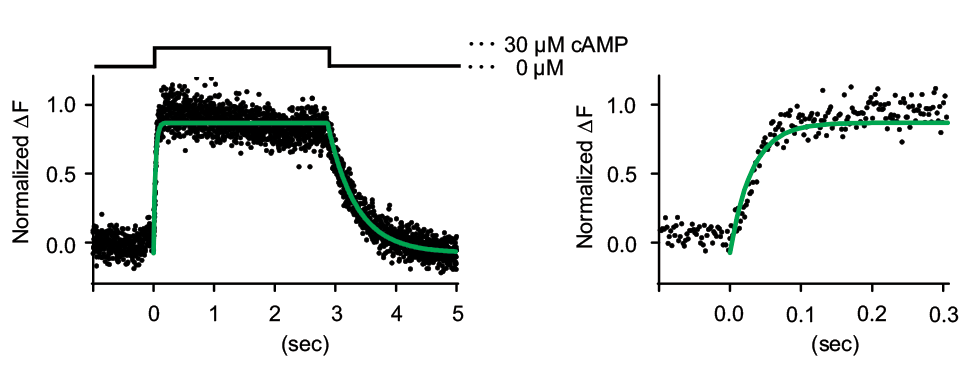
This indicator is highly specific to cAMP.
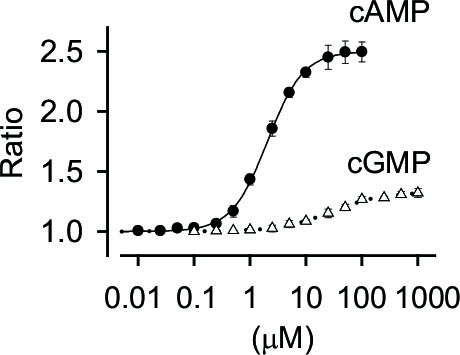
By conjugating with cpGFP and mCherry, this cAMP probe is reformed to ratio-metric probe usable for quantification of cAMP concentration.
This cAMP-specific indicator would be widely used as a standard probe in various neuronal systems together with the calcium indicator GCaMP.
4. Publications
4.1 Journals(OIST researchers are underlined)
Hori T, Eguchi K, Wang H-Y, Miyasaka T, Guillaud L, Taoufiq Z, Mahapatra S, Yamada H, Takei Y, Takahashi T (2022) Microtubule assembly by tau impairs endocytosis and neurotransmission via dynamin sequestrtion in Alzheimer’s disease synapse model. eLife 11, e73542.
Kawata S, Mukai Y, Nishimura Y, Takahashi T, Saitoh N (2022) Green fluorescent cAMP indicator of high speed and specificity suitable for neuronal live-cell imaging. Proc Natl Acad Sci USA 119, e2122618119.
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
Oral presentation
1. Tomoyuki Takahashi "Vesicular clearance and replenishment mechanisms at release sites in the central presynaptic terminals" 「中枢シナプス前末端放出部位における小胞除去・補充メカニズム」,
細胞システム理解のためのシグナル応答原理解明の最前線, National Institute for Physiological Sciences , Sept 15-16, 2022
2. Tomoyuki Takahashi “NO boosts anoxia-induced presynaptic LTP” in JSPS Core to Core program symposium "Molecular Physiology of Neuronal Signaling, Circuits and Behavior", Doshisha University, January 12-14, 2023
5. Intellectual Property Rights and Other Specific Achievements
Prof. Takahashi named 2022 IUPS Fellow
6. Meetings and Events
Nothing to report
7. Other
Invited lecture
1. Tomoyuki Takahashi ” Synaptic models of neurological diseases” in Luncheon Seminar, the 63rd Annual Meeting of the Japanese Society of Neurology, Tokyo International Forum, May 19, 2022
2. Tomoyuki Takahashi " Mechanisms underlying the action of volatile anesthetics「吸入麻酔薬の作用メカニズム」" the 18th Anesthesiology Summer Seminar Japan, Bankoku Shinryokan Resort MICE facility, July 23, 2022



