FY2015 Annual Report
Cellular and Molecular Synaptic Function Unit
Distinguished Professor (Fellow) Tomoyuki Takahashi

Abstract
The giant synapse calyx of Held visualized in rodent brainstem slice is an excellent preparation for studying presynaptic mechanisms, but it has some limitations including difficulty of molecular manipulations and inadequate transparency for imaging vesicles and presynaptic molecules. To break through these technical problems, we developed a calyx-like giant synapse preparation in dissociated culture. From newborn mice pups, we isolated tissues from cochlear region for presynaptic neurons and MNTB regions for postsynaptic neurons. After GFP-labeling presynaptic neurons, we co-cultured them in media containing various ingredients. After 10 days in culture GFP-labeled cochlear neurons extended their axons and formed calyx-like terminals surrounding postsynaptic neurons. These terminals underwent functional maturation and neurotransmitter release started after 15 days in vitro (DIV). They also underwent structural maturation with initial ring-like structures transformed later to finger-like structures attached with many swellings, like those described for the calyx of Held in vivo. To these calyx-like synapses in culture, we made it possible to apply imaging techniques thereby studying vesicle exocytosis and endocytosis using FM dye and transfected pHluorin, and monitoring vesicle trafficking using CypHer or quantum dot fluorescent labelling.
Dimitar Dimitrov, Hiroshi Takagi, Laurent Guillaud, Naoto Saitoh, Kohgaku Eguchi, Tomoyuki Takahashi (2016) J Neurosci 36, 3600-3610.
1. Staff
- Dr. Hiroshi Takagi, Group Leader
- Dr. Kohgaku Eguchi, Researcher
- Dr. Laurent Guillaud, Researcher
- Dr. Zacharie Taoufiq, Researcher
- Dr. Dimitar Dimitrov, Specialist, Technician
- Anna Garanzini, Technician
- Lashmi Piriya Ananda Babu, Graduate Student
- Ms. Larisa Sheloukhova, Lab Rotation Student (from January, 2016)
- Sayori Gordon, Research Unit Administrator
2. Collaborations
2.1 Theme: Regulatory mechanisms for transmitter release
- Type of collaboration: Joint research
- Researchers:
- Principal researcher: Professor Tomoyuki Takahashi, OIST
- Associate professor Tetsuya Hori, Doshisha University
- Dr. Manami Yamashita, Doshisha University
- Associate professor Naoto Saitoh, Doshisha University
- Name of partner organization: Doshisha University Faculty of Life and Medical Sciences
2.2 Theme:Effect of photo-switchable microtubules disassembler PST on α-synclein-induced inhibition of vesicle endocytosis
- Type of collaboration: Scientific Collaboration
- Researchers:
- Principal researcher: Professor Dirk Trauner, Ludwig-Maximillians-University
- Dr. Oliver Thorn-Seshold, Ludwig-Maximillians-University
- Name of partner organization: Department of Chemistry and Pharmacy and Centre for Integrated Protein Science, Ludwig-Maximillians-University
3. Activities and Findings
3.1 Reconstitution of giant mammalian synapses in culture for molecular functional and imaging studies (Dimitrov et al, 2016, J Neurosci ).

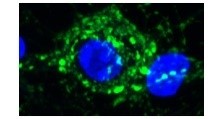
Cochlear nuclei (CN) were dissected from newborn mice and dissociated using trypsin. CN neurons were then labeled with GFP transfected by electroporation. Medial nuclei of trapezoid body (MNTB) were also dissected and neurons were dissociated for co-culture with CN neurons. During co-culture, GFP-labeled axons are often seen to grow and form calyx-like axo-somatic contacts with non-labeled MNTB neurons (higher magnification pictures in the middle panel and 3D picture with nucleus labeled blue with DAPI in the right panel). In situ, CN neurons form the giant synapse calyx of Held with MNTB neurons.Thus, this preparation apparently replicates the giant synapse in culture.
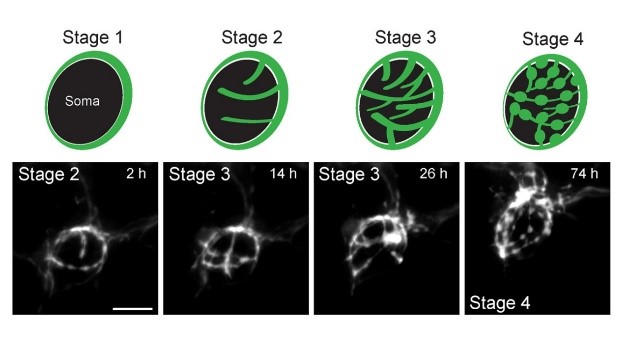
The calyx synapse in culture undergoes structural changes during culture; initial ring-like structure are branched (Stage 2, 3) and swellings are emerged at their ends (Stage 4).
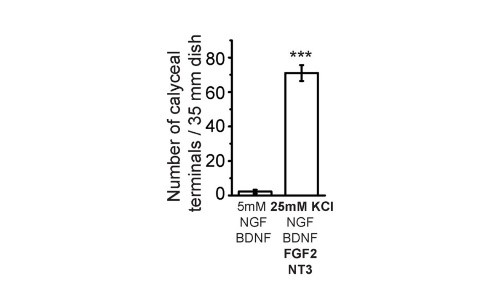
After trials and errors, we found an optimal composition of culture media to facilitate giant calyceal synapse. High concentration KCl (25 mM) helps to maintain neuronal condition and FGF2 for neuronal growth. Among them the most important and specific factor for giant synapse formation is neurotrophin 3 (NT3), without which giant calyceal synapses formation is minimal though small synapse formation is not much affected.
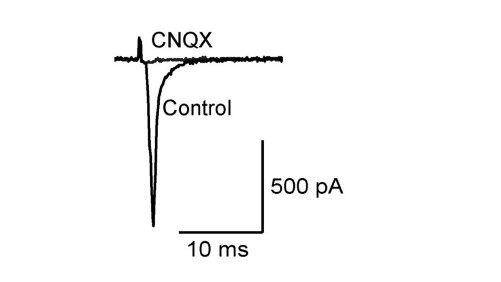
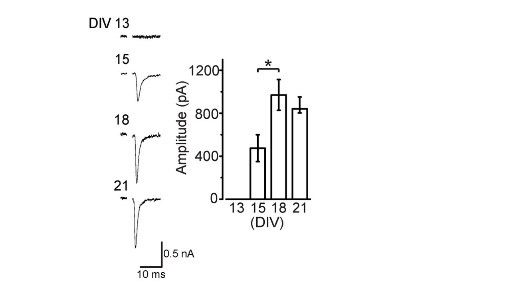
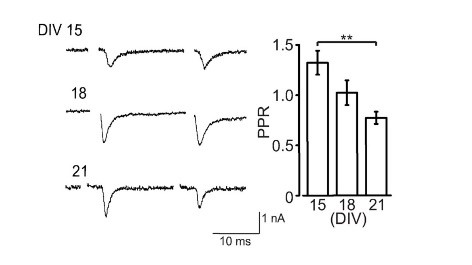
Like at the calyx of Held, calyceal synapse in culture is glutamatergic with its EPSCs blocked by CNQX. EPSCs become detectable at DIV15 and grow larger at DIV18. The paired pulse ratio (PPR; 2nd EPSC size relative to the 1st EPSC size) decreases during culture (right panel) implying that neurotransmitter release probability increases during development in culture.
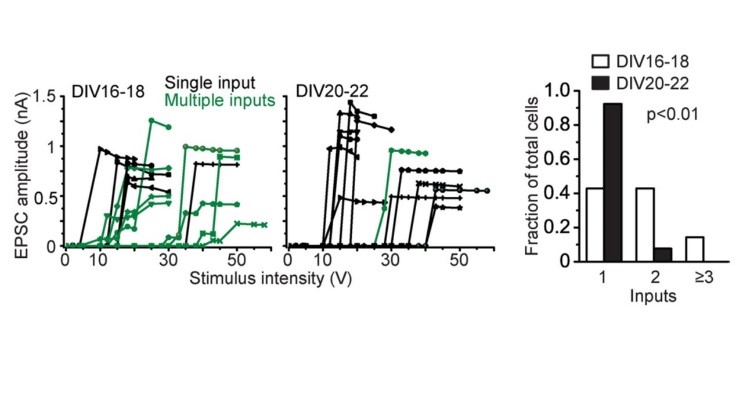
Just after calyceal synapse formation, a postsynaptic neuron receives multiple inputs (green) deduced from multi-step increase in EPSC amplitude in response to gradually increased stimulation intensity. During culture, these multiple inputs are eliminated into single input (black), showing all-or-none appearance of EPSCs to graded stimulation intensity. The size of EPSCs, developmental increase in release probability, synaptic elimination in cultured calyceal synapses altogether resemble those at the calyx of Held at postnatal day 4-5.
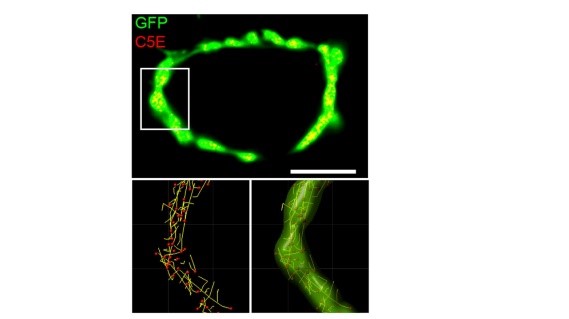
One important advantage of this giant synapse in culture is its feasibility for imaging studies. We took its advantage and performed synaptic vesicle (SV) real time imaging. We loaded synaptotagmin2 antibody-conjugated fluorescent probes into SVs during endocytosis to make SVs visible (yellow spots on the top panel) in calyceal terminals. At resting terminals, SVs move dynamically along various trajectories (bottom panels). Hence it becomes possible to analyze SV movements quantitatively (see below).
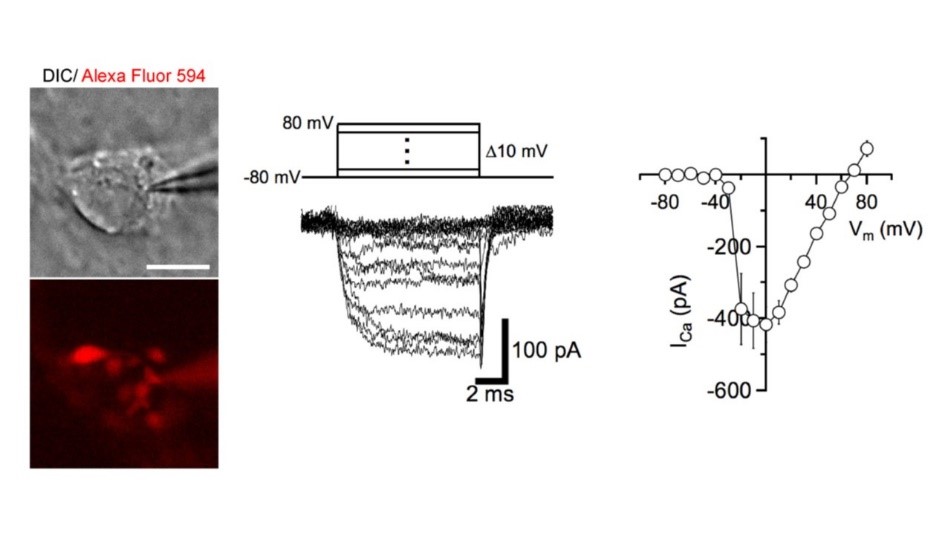
At these calyceal presynaptic terminals, 4t is possible to make whole-cell patch-clamp recordings, eg of presynaptic Ca2+ channel currents mediating neurotransmitter release.
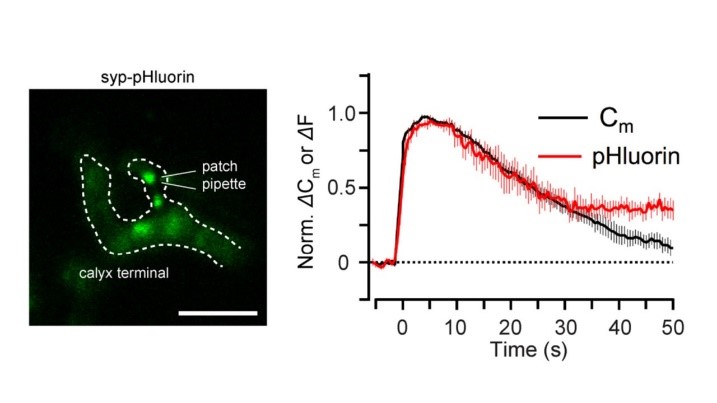
Capacitance measurements can be made at this presynaptic terminal, simultaneously with imaging. This enables one to directly compare the time course of SV endocytosis (Cm) and acidification (pHluorin).
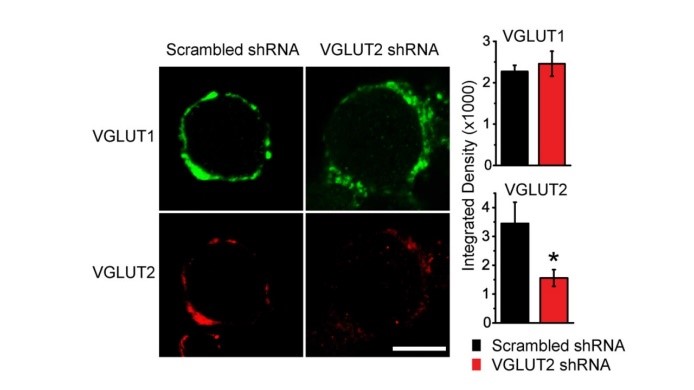
A great advantage of culture system is that expression of a particular protein can be attenuated by shRNA techniques. Viral infection of the shRNA of the vesicular glutamate transporter VGLUT2 in CN neurons significantly reduced VGLUT2 expression in calyceal terminals.

EPSCs recorded from these terminals were much smaller than controls infected with scrambled shRNA. Neither PPR nor the coefficient of variation (CV) showed a change, suggesting that the reduction of EPSCs reflects a reduction of vesicular, or quantal, synaptic response due to reduced magnitude of filling of SVs with glutamate or reduced number of SVs filled with glutamate.
3.2 Vesicle imaging in cultured calyx terminal (Guillaud et al, manuscript in preparation).
We characterized vesicle dynamics in the calyceal terminals in culture. In resting terminals labeled vesicles moved with irregular speeds and various trajectories. After fixation of the tissue with aldehyde, the SV mobility dropped down to 1/8, providing a good S/N ratio for SV imaging. Using IMARIS software, we analyzed SV movements quantitatively. SVs traveling a longer distance generally had a higher maximal velocity. The SV mobility in calyceal terminal swelling was twice higher than that in hippocampal presynaptic boutons when compared for similar size structures. Percentage of long SV trafficking (8 μm on average) decreases from 30% to 10% as presynaptic terminals undergo structural development from stage 1/2 to 3/4 (see above). VGLUT1-venus and VGLUT2-venus were transfected into separate CN neurons. VGLUT1-venus SVs showed higher mobility than VGLUT2-venus SVs. Furthermore, track length and max speed are higher and SVs with active directional modality was more frequently observed in the former SVs. These results suggest that SV signaturecan affect SV mobility. Disrupting microtubules disassembly using nocodazole preferentially reduced long/fast traffics, suggesting that microtubules are involved in long/fast SV trafficking. When vesicle exocytosis was triggered by bath-application of 65 mM [KCl] solution, many SVs underwent exocytosis, long/fast SV movements were significantly reduced, and remaining SVs became immobile. Hypertonic sucrose solution had no effect on SV mobility, but reduced directional modality of SVs. Electrical stimulation at 1 Hz had no appreciable effect.
3.3 Primary targets of diseases caused by excess endogenous presynaptic proteins (Eguchi et al, manuscript in preparation).
α-synuclein is an endogenous protein normally attached to vesicular or plasma membranes in the nerve -terminal. Excess α-synuclein is known to accompany α-synucleinopathy including Parkinson’s disease. We have loaded α-synuclein into calyx terminals in acute slices and found that it slows endocytosis with no immediate effect on exocytosis or ICa. This inhibitory effect of α-synuclein on vesicle endocytosis was rescued by co-loading the microtubules depolymerizing drug nocodazole in nerve terminals. Furthermore, a photo-switchable microtubules depolymerizer photostatin co-loaded with α-synuclein rescued the latter effect under 390 nm illumination, but its rescuing effect was reversed at 510 nm illumination. α-synuclein has little effect on basal neurotransmission, but slows recovery of EPSCs from short-term synaptic depression, thereby impairing fidelity of high frequency neurotransmission. Nocodazole rescued all these defects caused by α-synuclein. These results suggest that microtubules over-assembly underlie the loss of function caused by αsynuclein loading.
3.4 Proteomics of presynaptic proteins (Taoufiq et al, manuscript in preparation).
We developed ultra-high resolution mas-spectrum analysis and detected >5,000 proteins in synaptosomal fraction from P20 rat brain tissue. We further dissected synaptosomes into free vesicle, cytosol, membrane and mitochondrial fractions and quantitated copy numbers of individual proteins in each fractions. After confirming that typical vesicle proteins or active zone (AZ) proteins are predominantly in free vesicle or membrane fractions including AZ, we found many other proteins residing in both free vesicle and AZ membrane fraction in different quantities. We have also found new presynaptic proteins and unexpected expression patterns of known proteins.
3.5 Presynaptic molecular mechanism underlying ischemic long-term potentiation (Takagi et al, manuscript in preparation).
When brains are in ischemic state, neurons start to degenerate because of glutamate efflux from cells. Hippocampal CA1 region is known to be one of the most sensitive regions to ischemic insult. In fact, glutamatergic field EPSPs recorded from CA1 undergo long-term potentiation upon application of oxygen/glucose deficient (OGD) solution in hippocampal slices. This ischemic LTP (iLTP) is induced presynaptically because it accompanies a decrease in the PPR (see above). After experiments using pharmacological tools, we found that the presynaptic mechanism underlying iLTP largely overlap with that operating for exo-endocytic balancing at the calyx of Held (Eguchi et al, 2012 Neuron) as well at hippocampal synapses in culture (Micheva et al, 2003 Nat Neurosci). Namely, both the iLTP and exo-endocytic balancing mechanisms are mediated by a signaling molecule cascade including NMDA receptor, NO, protein kinase G, Rho kinase, and PIP2. On top of it our results suggest involvement of astrocyte in the upstream of NO.
4. Publications
4.1 Journals
- Dimitar Dimitrov, Hiroshi Takagi, Laurent Guillaud, Naoto Saitoh, Kohgaku Eguchi, Tomoyuki Takahashi (2016) Reconstitution of giant mammalian synapses in culture for molecular functional and imaging studies. J Neurosci 36, 3600-3610.
- Tomoyuki Takahashi (2015) Strength and precision of neurotransmission at mammalian presynaptic terminals. Proc Jpn, Acad, Ser B 91, 305-320. Review article.
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Eguchi, K., Excess α-synuclein impairs the fidelity of synaptic transmission at calyx of Held. JSPS Core-to-Core Symposium“Mechanism of synaptic transmission", Université Paris Descartes, 12 rue de I’ Ecole de Médecine, Paris, France. September 17-18, 2015
- Guillaud, L., Dimitrov, D., Takahashi, T., Synaptic vesicle trafficking in giant glutamatergic nerve terminals. Gordon Research Seminar 'Excitatory Synapses & Brain Function (GRS)' Salve Regina University. Newport, RI., U.S.A. June 6-7, 2015
- Zacharie, T., Villar-Briones, A., Sathish, S., Takahashi, T., Comparative synapse proteomics reveals new molecules potentially involved in brain plasticity and cognitive diseases. Gordon Research 'Conference 'Excitatory Synapses & Brain Function'. Salve Regina University. Newport, RI., U.S.A. June 7-12, 2015
- Guillaud, L., Dimitrov, D., Takahashi, T. Synaptic vesicle trafficking in giant glutamatergic nerve terminals. Gordon Research Conference 'Excitatory Synapses & Brain Function' Salve Regina University. Newport, RI., U.S.A. June 7-12, 2015
- Zacharie, T., Villar-Briones, A., Sathish, S., Takahashi, T., Comparative synapse proteomics reveals new molecules potentially involved in brain plasticity and cognitive diseases. OIST Computational Neuroscience Course(OCNC). OIST Seaside House., Okinawa, June 17, 2015
- Dimitrov, D., Takagi, H., Guillaud, L., Saitoh, N., Eguchi, K., Takahashi, T., Cultured mammalian giant synapse preparation for molecular functional and imaging studies. The 38th Annual Meeting of the Japan Neuroscience Society. Kobe Convention Center, Kobe, Japan. July 28-31, 2015
- Eguchi, K., Zacharie, T., Takahashi, T., Excess α-synuclein impairs the fidelity of synaptic transmission by slowing vesicle endocytosis at calyx of Held. The 38th Annual Meeting of the Japan Neuroscience Society. Kobe Convention Center, Kobe, Japan. July 28-31, 2015
- Eguchi, K., Zacharie, T., Takahashi, T., Acute presynaptic loading of a-syunclein impairs synaptic fidelity by slowing vesicle endocytosis at glutamatergic synapses. The 45th Annual meeting of Society for Neuroscience. McCormick Place, Chicago, IL, U.S.A., October 17-21, 2015
- Dimitrov, D., Takagi, H., Guillaud, L., Saitoh, N., Eguchi, K., Takahashi, T., Reconstitution of giant mammalian synapses in culture for molecular functional and imaging studies. JSPS Core-to-Core Symposium“Mechanism of synaptic transmission". Université Paris Descartes, 12 rue de I’ Ecole de Médecine, Paris, France. September 17-18,2015
- Ananda Babu, L.P., Guillaud, L., Eguchi, K., Takahashi, T., Functional implications of microtubule network in synaptic transmission. JSPS Core-to-Core Symposium “Mechanism of synaptic transmission". Université Paris Descartes, 12 rue de I’ Ecole de Médecine, Paris, France. September 17-18, 2015
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 Fraunhofer life sciences and OIST Joint workshop
- Date: April 9, 2015
- Venue: OIST Campus Center Bldg.
- Speaker: Professor Tomoyuki Takahashi, OIST
6.2 The East Asia Joint Symposium (EAJS) 2015
- Date: November 14, 2015
- Venue: OIST Campus Center Bldg.
- Speaker: Professor Tomoyuki Takahashi, OIST
6.3 Invited Seminar
- Date: June 12, 2015
- Venue: Harvard Medical School, Department of Neurology, Center for Life Science. Boston, U.S.A.
- Speaker: Dr. Zacharie Taoufiq, OIST
- Title: Decoding synapse proteomics: from neuronal deconstruction to functional reconstruction.
- Date: June 15, 2015
- Venue: New York University, Center for Neural Science.
- Speaker: Dr. Laurent Guillaud, OIST
- Title: Multi-tracking and spatio-temporal analysis of synaptic vesicles in giant glutamatergic synapses.
- Date: August 13, 2015
- Venue: Department of Neurobiology, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany.
- Speaker: Dr. Zacharie Taoufiq, OIST
- Title: Decoding Synapse Proteomics: from neuronal deconstruction to functional reconstruction.
- Date: October 23, 2015
- Venue: New York University School of Medicine, New York, U.S.A.
- Speaker: Dr. Kohgaku Eguchi, OIST
- Title: Excess alpha-synuclein impairs synaptic fidelity via slowing synaptic vesicle endocytosis at calyx of Held synapses.
6.4 OIST Seminar
- Date: April 17, 2015
- Venue: OIST Campus Lab1
- Speaker: Assistant Professor Mitsuharu Midorikawa, Doshisha University
- Title: Imaging exocytosis of single synaptic vesicles at a fast CNS presynaptic terminal
- Date: May 15,2015
- Venue: OIST Campus Center Bldg.
- Speaker: Senior Editor, Dr. Peter Stern, SCIENCE
- Title: The manuscript selection process at SCIENCE
- Date: September 2, 2015
- Venue: OIST Campus, Lab 1
- Speaker: Ms. Madhurima Dhara, University of Saarland
- Title: v-SNARE-based protein-lipid interactions in Ca2+ triggered exocytosis
- Date: September 29, 2015
- Venue: OIST Campus Lab1
- Speaker: Associate Professor Masayuki Mori, Kyoto University
- Title: Molecular insight into Ca2+/Calmodulin-dependent regulations in TRPC channels.
7. Other
7.1: Teaching class ‘Introduction to super resolution microscopy (nanoscopy): imaging beyond the diffraction limit’, part of the 'Emerging technologies in life sciences' course.
For the first year PhD students organized by Pr. Ichiro Maruyama, OIST
Date: July 24, 2015
Speaker: Laurent Gillaud



