Research
|
about the PI | members | research | join our lab | lab culture |
#Bioinformatics #Evolution #Structural-Biology #AI #Bioremediation #Cofactor-engineering #Microbiology
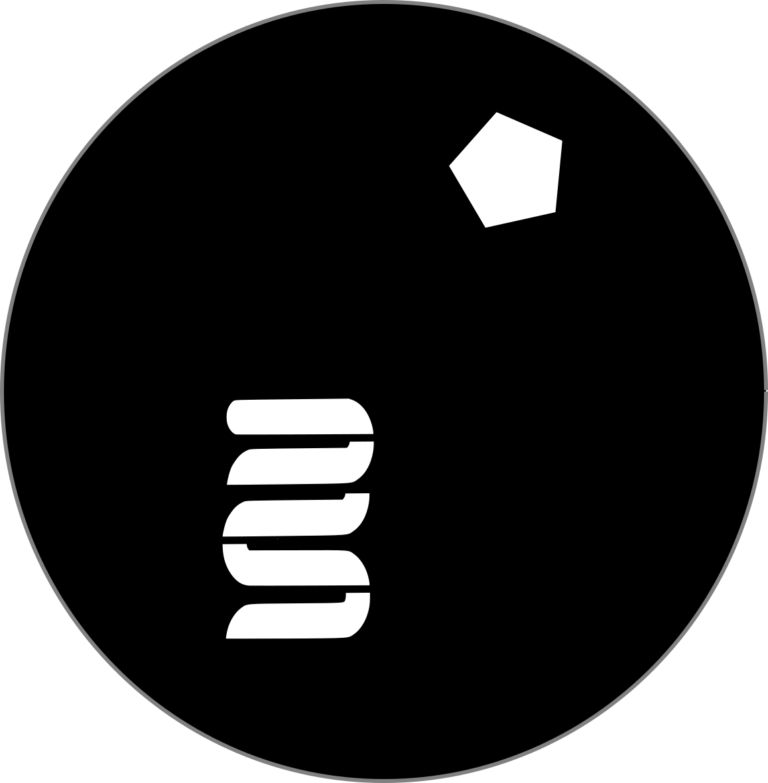
Design of proteins with electron transfer capabilities
Engineering protein wires for biocatalysis and bioremediation could enable faster and more controlled biochemical reactions. Such wires could allow for targeted redirection of electron flow within bacterial systems, improving the performance of bacterial factories and supporting energy-intensive processes without disrupting core metabolism. This technology is crucial for advancing biofuel production, bioremediation, and other sustainable applications by optimizing the catalytic efficiency and energy management of engineered microbes.
In nature, cells function as microscopic factories, utilizing energy to produce essential molecules for survival and growth through complex molecular machines called enzymes. As bioengineers, we enhance these natural processes by introducing artificial enzymes to create valuable products like medicines and fuels. However, this approach faces a challenge: competition for energy between the cell's normal functions and the new, added machinery.
Our lab aims to overcome this by developing cells equipped with enzymes that can receive energy from external power sources. This would allow cells to manufacture desired chemicals without depleting energy from the essential processes of the host.
Central to our approach are proteins with electron transfer capabilities to conduct electricity into the cell to overcome bottlenecks in sustainable production of industrial feedstocks and enable the creation of fuels and value-added chemicals directly from carbon dioxide.
To unravel the mechanistic secrets of natural and engineered proteins, we employ advanced techniques like X-ray crystallography and electron microscopy. By understanding how proteins evolve, we are paving the way to create novel proteins with customized properties for a wide range of biotechnological applications.
Studying the evolution of thead-like stinging proteins in cnidaria
The evolution of protein threads, such as the stinging wires of nematocysts, represents a remarkable example of protein innovation driven by ecological pressures like predation and defense.
These structures likely emerged from ancestral fibrous proteins, which were gradually adapted for specialized mechanical functions through processes like gene duplication and modular evolution. Over time, natural selection favored proteins with increased elasticity and strength, enabling the rapid, forceful discharge required to capture prey and deliver venom. Studying the molecular evolution and biomechanics of these protein threads offers valuable insights into the broader mechanisms of protein adaptation and functional diversification.
Previous work
InDels mediating coenzyme-binding divergence
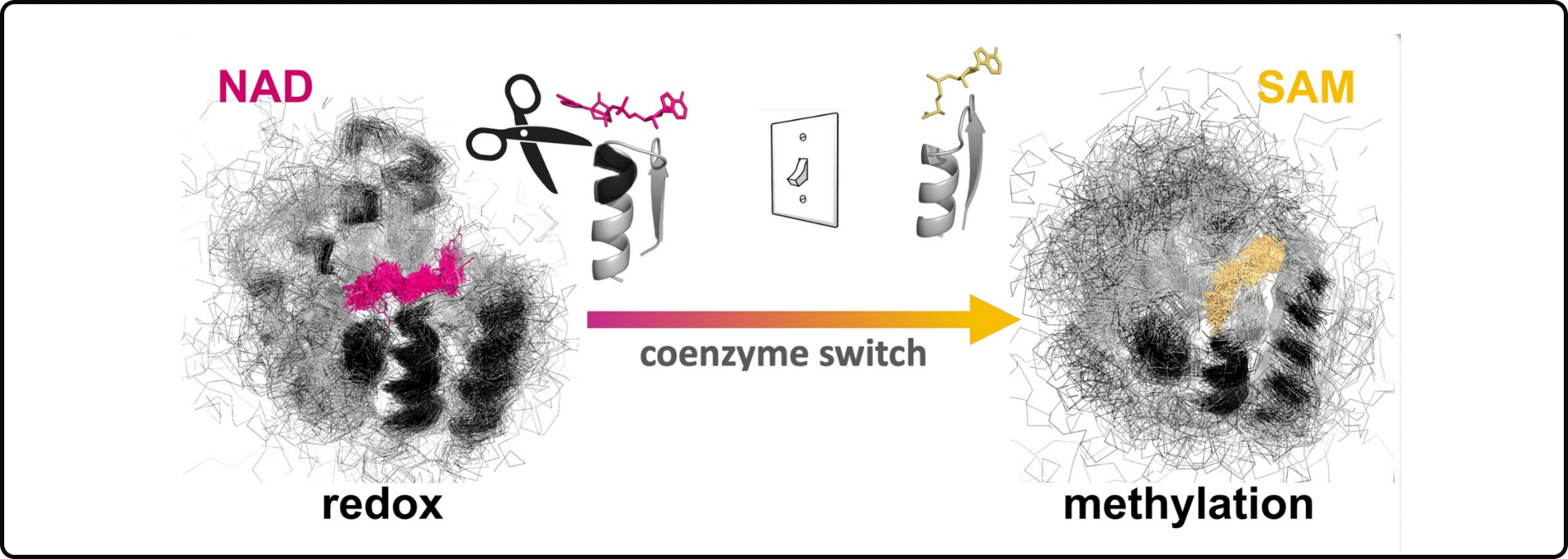
In this study, we focused on understanding how the most catalytically-diverse enzymes (Rossmann enzymes) switch their coenzyme preferences.
Enzymes are the molecular machines that facilitate chemical reactions in our cells, and coenzymes are small molecules that help enzymes do their job. We removed three specific amino acids (the building blocks of proteins) from Rossmann enzymes to switch the binding of a redox coenzyne towards a methylating one. These findings demonstrate that enzymes likely explored distinct chemistries with minor changes in their amino acid sequences.
InDels mediating structural transformations
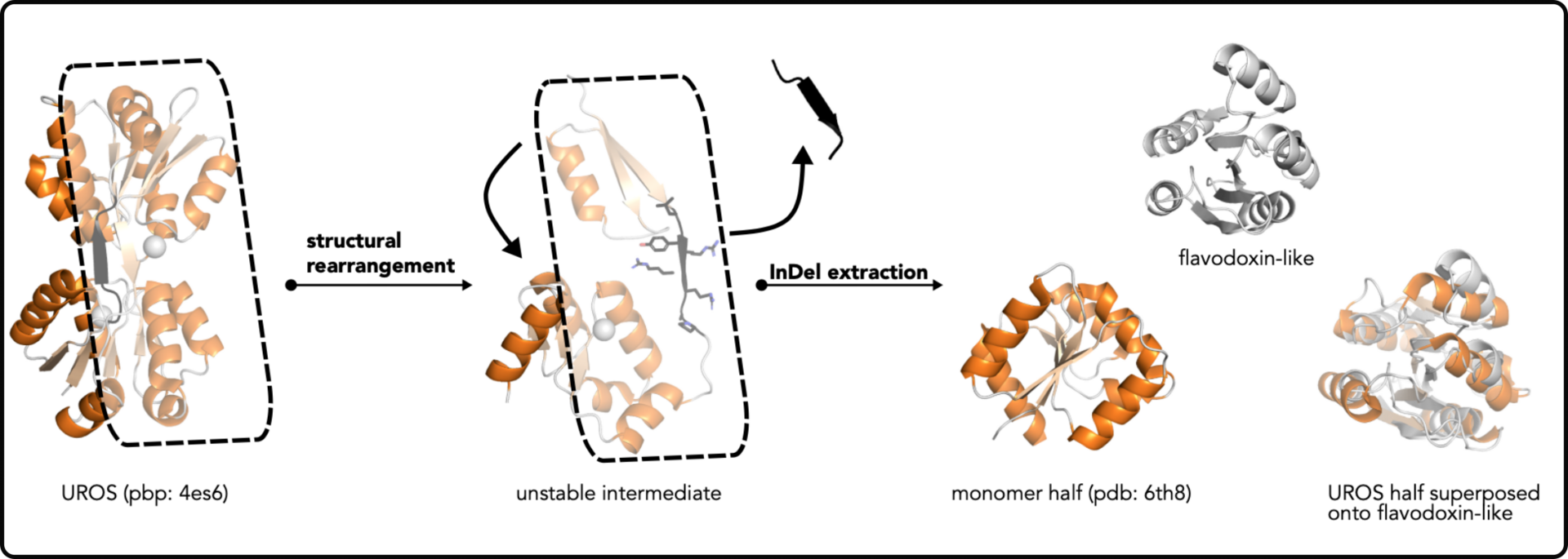
In this study, we explored how different protein structures, evolved from common ancestors.
Proteins are made up of a limited number of building blocks, and their diverse functions and shapes come from combining these building blocks in various ways.We discovered evidence suggesting that a specific protein fold, called HemD-like, emerged from another fold called flavodoxin-like, through a process involving gene duplication and segment swapping. To test our hypothesis, we reversed these evolutionary steps, recreating the flavodoxin-like shape from a HemD-half. This experimental result strongly supported our idea of a common ancestry for these two folds (Toledo-Patino, Biochemistry 2019).
Protein-LEGO finding the puzzles of Nature
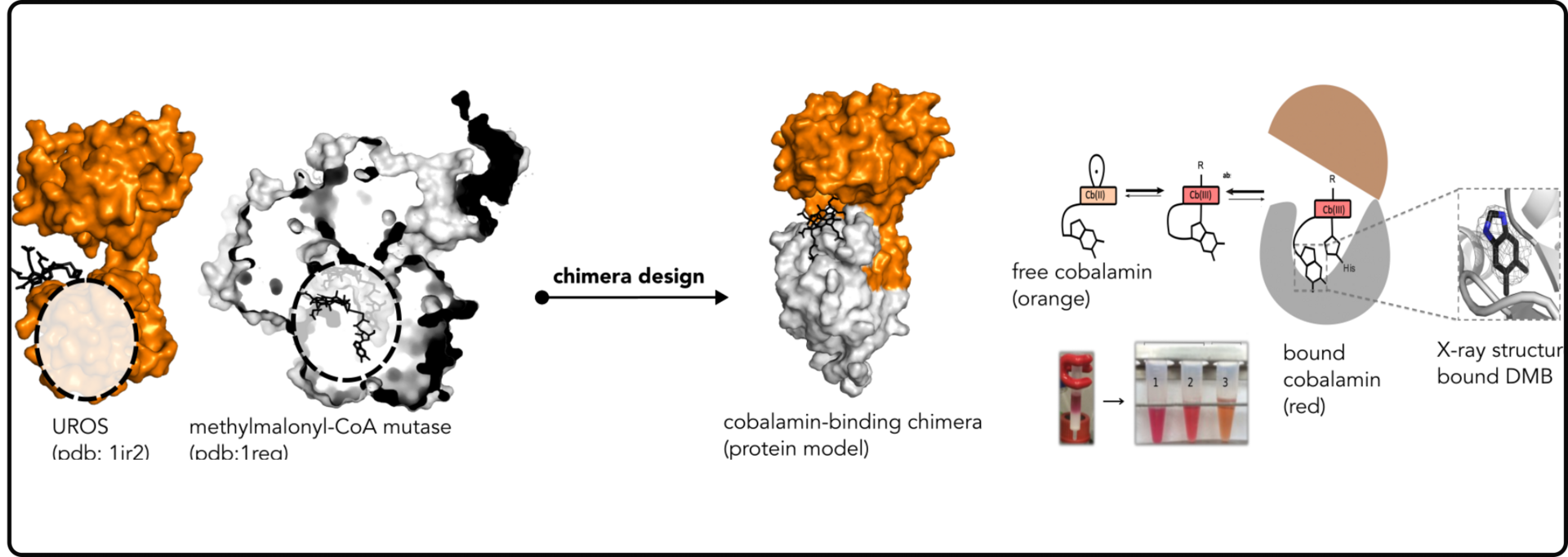
In this study we systematically searched for common fragments between proteins with distinct overall architecture, following the hypothesis that nature employed a “Lego-brick” strategy to build multiple architectures.
We discovered fragments that are present throughout the protein universe, appearing in diverse environments. Remarkably, we identified over a thousand sub-domain sized fragments that Nature has ingeniously repurposed to create new proteins (Ferruz et al, JMB 2020). These fragments offer an exciting and innovative avenue for protein design (Toledo-Patino et al, FEBS Letters 2024 & Toledo-Patino 20219).
|
|
top | about the PI | members | research | join our lab | lab culture |