FY2012 Annual Report
Brain Mechanism for Behaviour Unit
Professor Gordon Arbuthnott
Research Theme: The corticostriatal system in vitro

Abstract
We have exciting new results that are being prepared for publication with early versions having been presented at two international meetings during 2012. Dr Violeta Lopez Huerta has provided evidence for a synaptic underpinning of the grouping of neurons that fire in association in cortical cultures. Drs Lopez Huerta and Garcia Munoz have obtained pharmacological evidence for the importance of non-synaptic NMDA receptors in corticostriatal systems in vitro. Morever, Dr Omar Jáidar has good evidence that both groups of striatal output neurons are closely involved in the effects of dopamine depletion in the striatum and Dr Takuya Hikima also has convincing evidence that silent synapses of cortical axons can be awakened, independent of actions on calcium trafficking, by protein kinase A. After developing a method of growing in cultures dissociated thalamic and striatal neurons in different compartments Dr Marianela Garcia Munoz is now obtaining compelling multielectrode array recordings.
Collaboration with scientists in OIST and abroad has been rewarding as well. In OIST our Unit works closely with several scientists and enjoy fruitful meetings of the minds and interesting research. Experiments performed with Dr Beulah Leitch of the University of Otago, New Zealand, resulted in the publication of a paper in Neuroscience that suggests novel therapeutic strategies for absence epilepsy.
Collaboration with Professor Wing Ho Yung from the School of Biomedical Sciences, Chinese University of Hong Kong resulted in a high profile publication in Neuron providing evidence for the importance of cortical activation in Deep Brain Stimulation, which is used primarily for treatment of parkinsonism in drug refractory patients.
Collaboration with Professor William Staines (University of Ottawa, Canada) and Stephan Theiss (Heinrich Heine University of Dusseldorf, Germany) continued with a visit by Prof. Arbuthnott to Ottawa in early 2012 and the submission of a grant to the Strategic Japanese‐German Cooperative Programme on “Computational Neurosci-ence” with Stephan Theiss that only just missed being funded.
1. Staff
- Dr Gordon Arbuthnott, Professor
- Dr Marianela Garcia Munoz, Group Reader
- Dr Takuya Hikima, Researcher
- Dr Violeta Gisselle Lopez Huerta, Researcher
- Dr Omar Pedro Jaidar Benevides, Researcher
- Ms Malgorzata Broszkiewicz, Research Assistant (July 23 - September 21, 2012)
- Ms Sakurako Watanabe, Graduate Student (September - December, 2012)
- Ms Hiroko Chinone, Research Administrator
2. Collaborations
- Theme: Isolating genetically marked neurons in culture
- Type of collaboration: Joint research
- Researchers:
- Professor, William A. Staines, University of Ottawa, Canada
- Professor, Anthony Krantis, University of Ottawa, Canada
- Dr Sarah Schock, University of Ottawa, Canada
- Theme: Immunolocalization of proteins (L-type calcium channels [CaV1.3] and neurotransmitter receptors) in brain at electron microscope resolution.
- Type of collaboration: Joint research
- Researchers:
- Dr Beulah Leitch, Universtiy of Otago, New Zealand
- MSc Olga Shevtsova, University of Otago, New Zealand
3. Activities and Findings
We have worked on murine neurons from cortical, striatal and thalamic areas in culture and brain tissue slices. The experiments all were concentrated on the substrate on which dopamine acts in brain.
3.1 Short-term changes in neuronal plasticity associated with neuronal assembly formation in cortical cultures.
Dr Lopez-Huerta carried forward experiments on the mechanisms of assembly of groups of cortical cells that fire in concert in calcium imaging videos of cortical cultures. Additionally, by simultaneous whole cell patch recordings of neuronal pairs, it was possible to identify differences in the synaptic activity between two groups of neurons: those with a concomitant firing (< 10 ms between the first spike in a bust) and those with a sequential firing (> 10 ms between the first spike in a bust). Pairs of neurons firing concomitantly were distinguished by connections that showed short-term depression of synaptic responses. Sequential firing was characterized by synapses that displayed short-term facilitation (Fig. 1). In the literature, short-term facilitation is associated with smaller release probabilities while depressing synapses are associated with higher release probabilities. Low extracellular calcium, i.e., low probabilities of release, resulted in the generation of sequential activity patterns. Conversely, when the extracellular calcium was high, i.e., high probability of release, a drastic reduction in the network dynamics with a dominant state that engaged the activity of most neurons in the network was observed (Fig. 2).
These dramatic results caused quite a stir at the meeting in which they were presented. A manuscript is in the late stages of preparation describing this innovative method of modifying the formation of cell assemblies in these cultures.
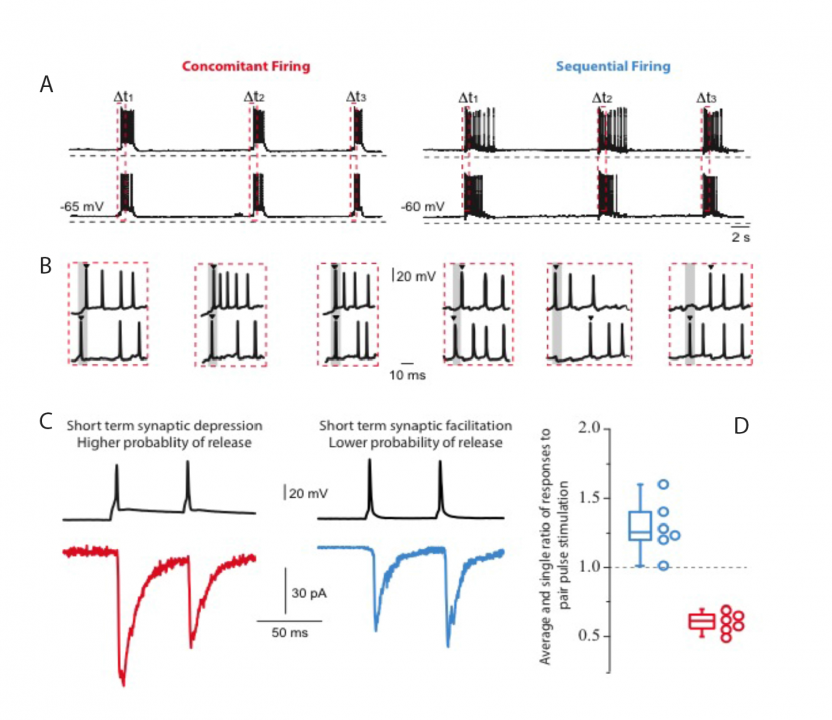
Figure 1:Concomitant and sequential firing patterns were revealed by simultaneous whole cell patch recordings of cultured cortical neurons. A) Concomitant firing is defined by action potentials recorded in a set of neurons with less than 10 ms time difference. Sequential firing is defined by action potentials recorded in a set of neurons with more than 10 ms time difference. Red dashed vertical rectangles indicate the start of a burst. B) Illustration of the two types of neuronal discharges. The gray area indicates 10ms from the start of the first spike of the group. C) Double pulse stimulation revealed the presence of short-term synaptic depression and short-term synaptic facilitation for concomitant and sequential discharges, respectively. D) Histogram of the average and individual values of the ratio of responses to pair pulse stimulation (n = 6 pairs in each group).
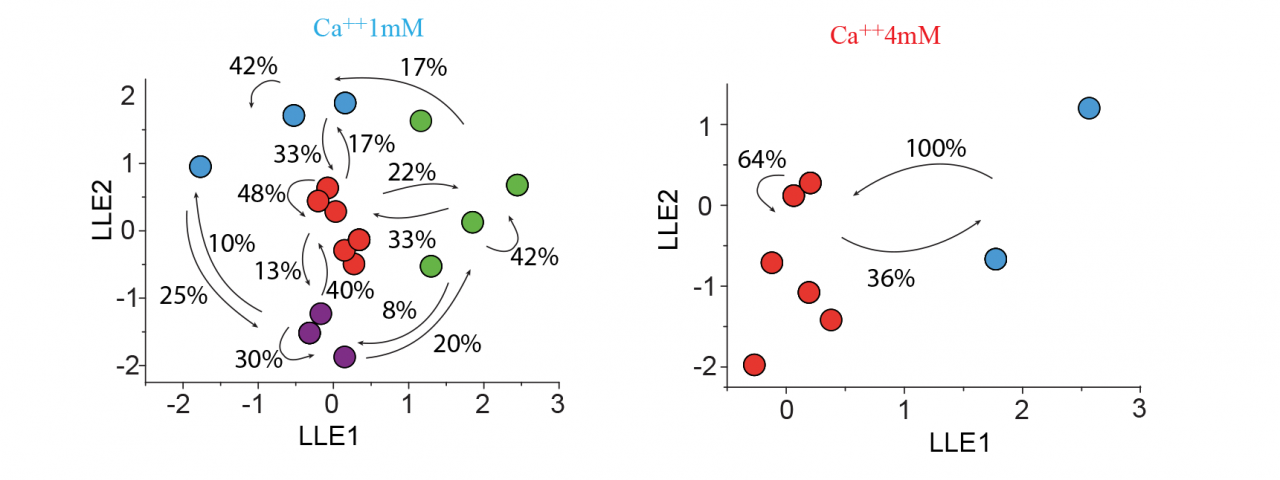
Figure 2: Different network activity of cultured cortical cells is observed under low and high external calcium concentrations.
A multidimensional reduction of vectors (local linear embedding -LLE) applied to the Fluo-4 optical recordings revealed different network activity when external calcium concentrations were modified. Under low extracellular calcium conditions (1 mM), and therefore under a low probability of release, diverse cyclic folds with compositional capabilities and the generation of diverse sequential activity patterns were observed. Following an increase in external calcium concentration (4 mM) and therefore inducing a high probability of release the network reduced the diversity of cell assembly. The presence of a dominant state (red) engaging most neurons suggests a high level of synchronicity at the onset of bursting activity and a network unable to generate sequential activity patterns.
3.2 Activation of presynaptically silent synapses by protein kinase A (PKA).
We have previously reported a marked increase in the release of synaptic vesicles imaged with SynpHluorin after the action of PKA generated by application of forskolin. We noticed that the average increase after PKA activation consisted of an increase in release from some synapses plus additional release from silent synapses (i.e., not releasing neurotransmitter) in the control state. These ‘presynaptically’ silent synapses could be activated by strong stimulation but they were not activated by our usual stimulation with 5 pulses at 20Hz. In many neurotransmitter systems, a fraction of presynaptic terminals fails to release vesicles in response to action potential stimulation and strong calcium influx. In a series of experiments Dr. Hikima has confirmed that PKA modulates the number of silent presynaptic terminals. Consistently, the action of forskolin is dependent on PKA and is independent of the extra calcium influx that can follow such a drug treatment. Further experiments suggest that PKA activation makes vesicles accessible in the releasable pool. Such an action at cortical synapses may underlie some of the long-term changes in presynaptic efficacy that have been demonstrated in cortical synaptic physiology.
3.3 Extrasynaptic NMDA receptors and the UP states in striatal neurons.
Extrasynaptic neurotransmission represents a subtype of volume transmission, taking place in the extracellular fluid of local circuits. It refers to the release and diffusion of neurotransmitters from the soma, axon or dendrites of neurons or after transmitter leak-out from the synaptic cleft. Extrasynaptic neurotransmission is important since it can modulate whole neuronal populations and their connectivity. We studied the effects of extrasynaptic glutamate receptors on the NMDA-induced re-awakening of depolarized up-state events in corticostriatal slices and cortical and striatal neurons plated in different compartments (Fig. 3). We hypothesized that extrasynaptic receptors were responsible for the return of the depolarized states in corticostriatal slices following prolonged NMDA receptor stimulation. Adding NMDA to either cultures (300nM) or slices (10µM) of mouse corticostriatal systems resulted in more spontaneous activity that is blocked by memantine, a potent extrasynaptic NMDA receptor blocker. Moreover, memantine caused a time-dependent acceleration of the decay of a typical NMDA current induced by train stimulation (20Hz) (Fig. 4)
One possible way to increase glutamate outside synapses is to hinder the activity of the glutamate transporter in glial cells. The research project of our first OIST graduate student on rotation Ms. Sukurako Watanabe consisted of whole-cell patch recordings of cortical neurons in culture (>15 DIV). In the presence of the glial glutamate uptake inhibitor dihydroxykainate, she observed a drastic increase in activity (Fig. 5; N=1) that suggests a possible important role of the glutamate transporter in striatal network activity. Obviously more experiments are needed.
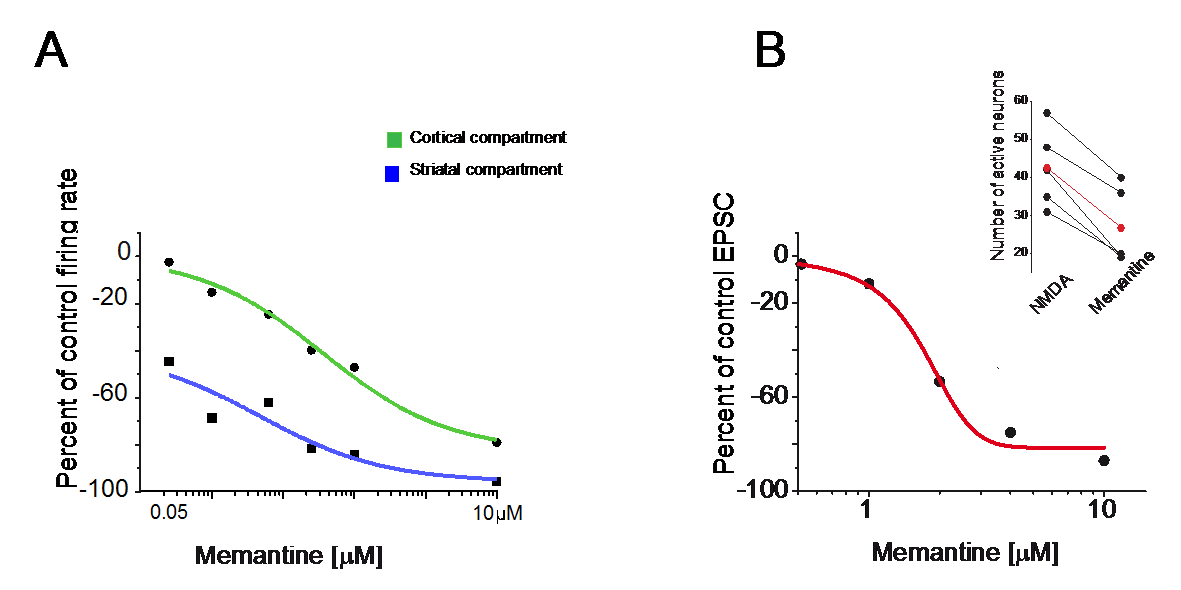
Figure 3:Blockade of extrasynaptic NMDA receptors with memantine reduced overall spontaneous activity.
A. Dose response curve for the effects of memantine (0.05, 0.1, 0.25, 0.5, 1 and 10 µM) of cortical and striatal neurons grown in different compartments. The decrease of NMDA induced activity was more pronounced for the striatal compartment.
B. Dose-response curve to memantine (1, 2, 4, 10µM) applied to striatal neurons recorded in slices (in Mg++ free medium, a holding potential at the chloride reversal potential -70mV and in the presence of DNQX). Insert indicates the general decrease in spontaneous NMDA-induced activity observed in slices after memantine (10µM) administration.
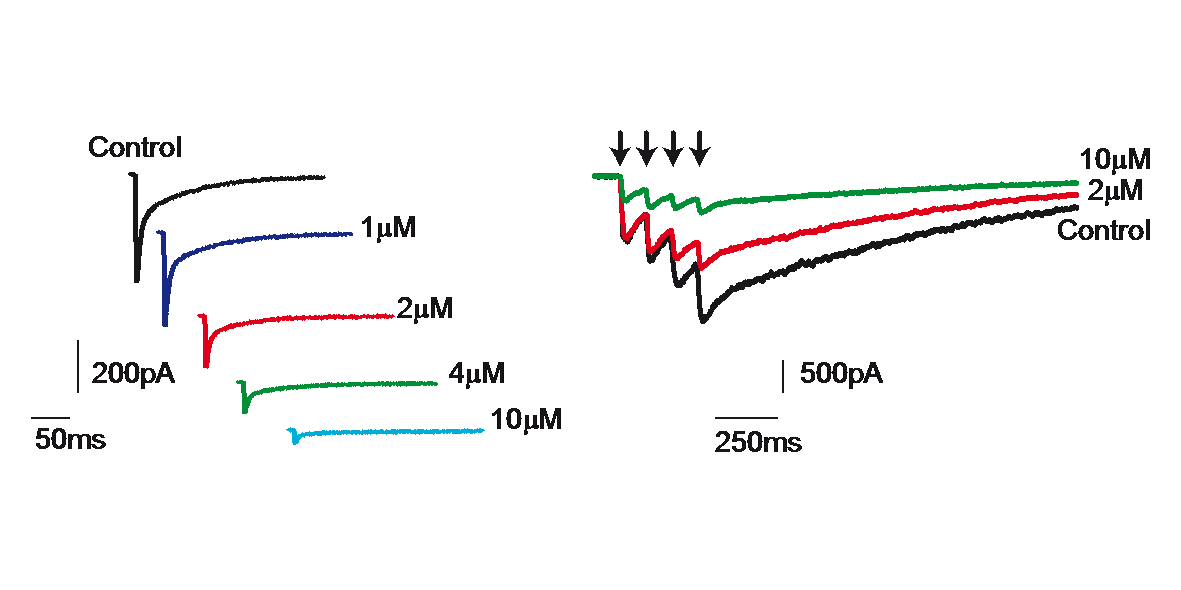
Figure 4: Blockade of NMDA receptor mediated current by memantine.
Delivery of a train of four stimuli (20 Hz) to striatal neurons recorded in slices produced the typical NMDA-induced long lasting postsynaptic response (control). Blockade with memantine (2 and 10µM) of the NMDAR-mediated current confirmed the participation of extrasynaptic receptors.
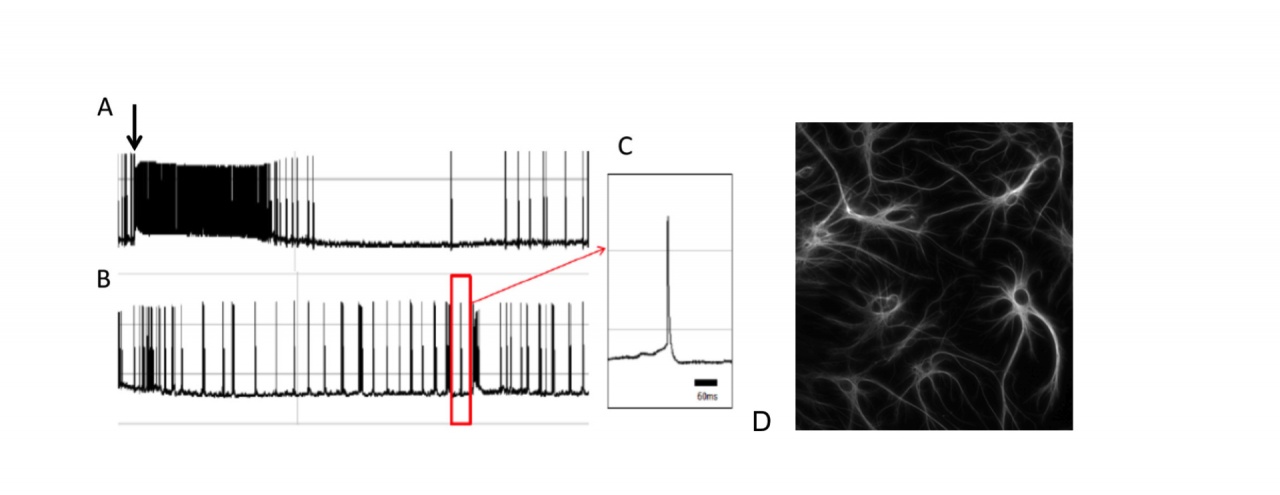
Figure 5: Depolarizing effect of extracellular glutamate accumulation on a cortical neuron following administration a glial glutamate uptake inhibitor.
A. Arrow indicates administration of dihydrokainate (250µM). B. Recordings following the drug washout. C. A single spike. D. Glial cells in a similar culture of cortical neurons stained with antibodies to glial fibrillary acid protein (GFAP).
3.4 Dopamine receptor D1 and D2 expressing neurons are equally involved in effect of dopamine depletion.
Since Dr. Jáidar joined the Unit he has been using calcium imaging of striatal tissue slices of bacteria artificial chromosome (BAC) transgenic mice expressing enhanced GFP under the control of D1 and D2 receptor promoters. Prior to calcium imaging experiments (9-14 days) a unilateral 6-hydroxydopamine injection (0.5 µl) into substantia nigra compacta was performed to deplete the striatum of dopamine. To estimate the extent of the dopamine depletion animals were tested for turning following apomorphine (0.1mg/kg, subcutaneous) at least 48 h before recordings. The results show that dopamine depletion resulted in an increase in striatal medium spiny neuronal activity independent of the neuronal receptor type (Fig. 6). Considering that the methods to detect the neuronal groups with calcium imaging techniques cannot inform the amount of action potentials being produced by the neurons it is not possible to discern subtleties between the firing properties of D1 and D2 receptor-expressing neurons, but it is clear that both groups are involved in the consequences of dopamine depletion.
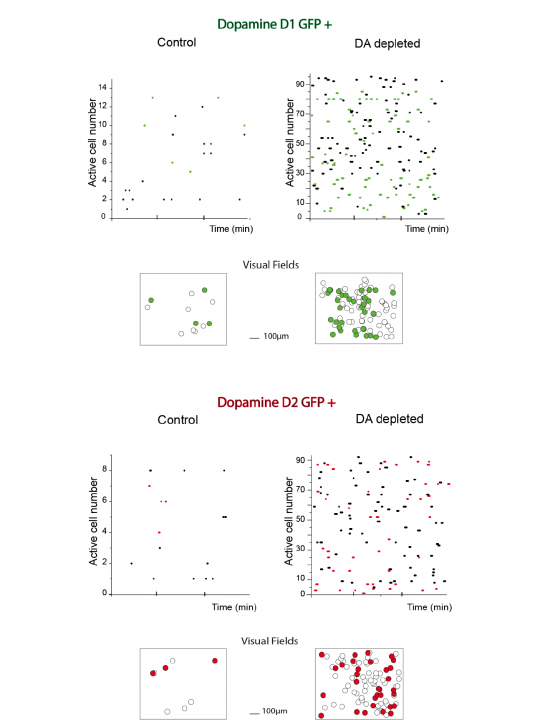
Figure 6: Depletion of dopamine enhances neuronal network activity of both types of medium spiny neurons.
Neuronal activity was evaluated by the records of intracellular calcium signaling (Fluo-4). A single comparison of raster plots of neuronal activity between naïve and dopamine depleted animals is presented for neurons with enhanced GFP expression under the control of D1 (n=20, green, top) and D2 receptor promoter (n=20, red, bottom). Representations of the visual field images are also shown.
3.5 Research performed in collaboration: Collaborations within OIST
- Physics and Biology Unit at OIST under the leadership of Prof Jonathan Miller to examine more efficiently the electrical activity in the MEAs.
- Optical Neuroimaging Unit at OIST under the leadership of Prof Bernd Kuhn to examine, in awake mice, the output pathway from thalamus to cortex that is the final output pathway from the basal ganglia. We have the first images of activity in the thalamocortical fibres in freely moving mice thanks to the project work of an OIST graduate student Mr. Hiroaki Hamada.
3.6 Research performed in collaboration: Collaborations outside OIST
- Prof William Staines and his group at Faculty of Medicine, University of Ottawa, Canada. Another paper is in preparation for publication in 2013.
- Stephan Theiss at the Institute of Clinical Neuroscience, University Heinrich-Heine, Dusseldorf, Germany. We nearly gained a Japan/Germany collaborative grant. We hope we can cross the border to funding in 2013.
- Prof Wing Ho Yung from the School of Biomedical Sciences of the Chinese University of Hong Kong. We are now looking into another exiting topic: the recovery from the effects of dopamine loss in freely moving rats (Li et al, 2012).
- Dr Beulah Leitch and her students at the Department of Anatomy, University of Otago, New Zealand. The ‘Stargazer’ mouse has an epileptic phenotype that resembles ‘absence’ epilepsy in children. We were able to show the dramatic reduction in excitatory amino acid receptors in the thalamic reticular nucleus that may cause the cortical hyper-excitability that could underlie the absence seizures (Barad et al, 2012).
- Dr Urs Frey at the Frey Initiative Research Unit, Quantitative Biology Center in Riken Kobe, Japan. We have begun to explore the use of his complementary-metal-oxide-semiconductor (CMOS) integrated circuit for amplification, addressing and analog-to-digital conversion of signals from neurons that connect together on multi array electrodes. Although the surface is not the best for cell culture we are exploring ways to attach the cells to the integrated circuit surface.
4. Publications
4.1 Journals
- Barad, Z., Shevtsova, O., Arbuthnott, G. W. & Leitch, B. Selective loss of AMPA receptors at corticothalamic synapses in the epileptic stargazer mouse. Neuroscience 217, 19-31, doi:10.1016/j.neuroscience.2012.05.011 (2012).
- Li, Q. et al. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 76, 1030-1041, doi:10.1016/j.neuron.2012.09.032 (2012).
- López-Huerta VG, B.-H. E., Bargas J, Galarraga E. Presynaptic modulation by somatostatin in the rat neostriatum is altered in a model of parkinsonism. Journal of Neurophysiology 108, 1032-1043, doi:10.1152/jn.00244.2012 (2012).
- López-Huerta VG, C.-R. L., Galarraga E, Tapia D, Fiordelisio T, Drucker-Colin R, Bargas J. The balance of striatal feedback transmission is disrupted in a model of parkinsonism. Journal of Neuroscience 33, 4964-4975, doi:10.1523/JNEUROSCI.4721-12.2013. (2013).
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Arbuthnott, G., Lopez Huerta V. G. & Garcia Munoz M.A Role For Non-Synaptic NMDA Receptors In Striatal Burst Firing Patterns. FENS Forum of Neuroscience (Barcelona, Spain, 2012).
- Arbuthnott, G. W. et al.Facebook for neurons? Helping isolated neurons make appropriate connections. The Physiological Society (Edinburgh, Scotland, 2012).
- Arbuthnott, G. W. et al. Helping isolated neurons make appropriate connections. The Joint Annual Scientific Meeting of the Hong Kong Society of Neurosciences and the Biophysical Society of Hong Kong (Hong Kong,2012).
- Hikima, T. & Arbuthnott, G. Evaluation of activating presynaptically silent synapses using fluorescent pH reporter measurements. The 35th Annual Meeting of the Japan Neuroscience Society (Nagoya, Japan, 2012).
- Hikima, T. & Arbuthnott, G. W. Evidence for the activation of presynaptically silent cortical synapses using a fluorescent probe. Society for Neuroscience (New Orleans, U.S.A., 2012).
- Hikima Takuya, A. G. Fluorescent pH Reporter Measurements Of Vesicular Release From Terminals Of Cortical Neurons. FENS Forum of Neuroscience (Barcelona, Spain, 2012).
- Jaidar, O., Carrillo-Reid, L. A., Arbuthnott, G. W., Gargas, J. & Hernandes, A. Contribution Of Direct And Indirect Pathways During The Dynamics Of Parkinsonian Microcircuit. FENS Forum of Neuroscience (Barcelona, Spain, 2012).
- Jaidar, O. P., Lopes-Huerta, V. G. & Arbuthnott, G. W. Modification of striatal network dynamics by activity in fast spiking interneurons. Society for Neuroscience (New Orleans, U.S.A., 2012).
- Lopez-Huerta, V. G., Carrillo-Reid, L., Garcia-Munoz, M., Theiss, S. & Arbuthnott, G. W. Sequential activity patterns encoded by cell assemblies in cortical networks. Society for Neuroscience (New Orleans, U.S.A., 2012).
- Yung, W., Ll, Q., Ke, Y. & Arbuthnott, G. W. Interference of pathological activities in motor cortex by antidromic spikes during deep brain stimulation in freely moving parkinsonian rats. Society for Neuroscience (New Orleans, U.S.A., 2012).
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 Seminar
- Date: March 5, 0213
- Venue: OIST Campus Lab1
- Speaker: Prof Wing Ho Yung (School of Biomedical Sciences, Chinese University of Hong Kong)



