Posts
Three more papers this year!
Three more papers came out this year: from Sebastien, Abir and Yu-Tao, and Ayumu. Congratulations to all CCCU members! Our work was also featured on the front cover of Organometallics!
Our paper is featured on the cover of ChemCatChem!
See Cover Feature of ChemCatChem for the Special Issue: Women of Catalysis
Here is the full graphics (thanks to skeppuccino!):

New Chem. Comm. paper by Shubham and Eugene is online now!
Read about unusual rearrangement of the PNP ligands in Ru complexes here.
Congratulations to Sebastien for CSJ Student Presentation Award!
We just received good news today that Sebastien got the 2019 Student Presentation Award at the 99th Chemical Society of Japan meeting! Congratulations for great presentation!
ChemCatChem paper online!
Our paper in ChemCatChem is online, and it will be a part of the Special Issue on "Women of Catalysis"!

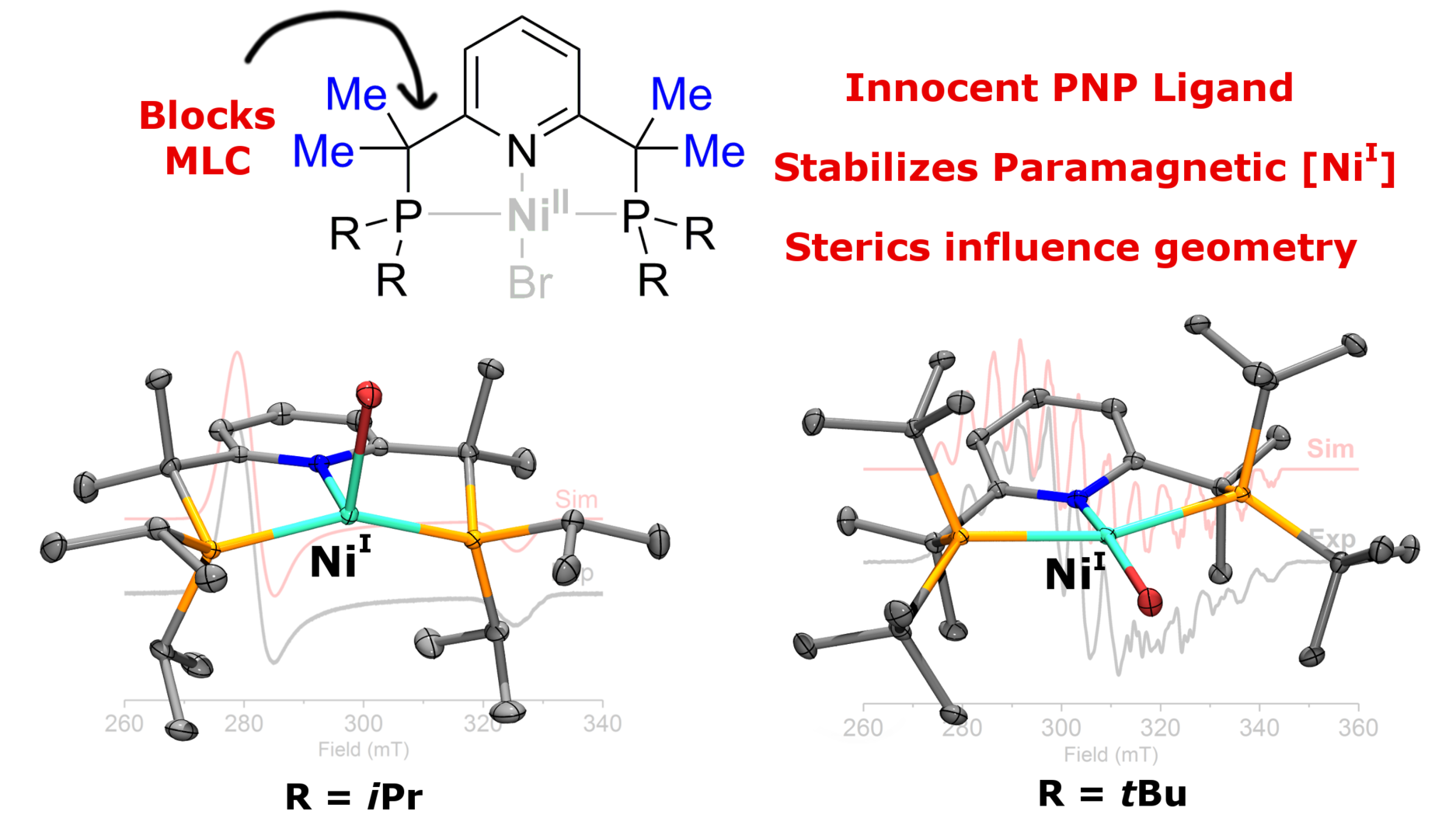
Congratulations to Sebastien with his first paper!
Sebastien's first paper as the first author is now online as an ASAP article in Organometallics! Read here about unusual paramagnetic Ni(I) complexes with the sterically hindered pincer ligands.
New paper in Chem. Comm.!
Congratulations to Abir, Pradnya, Hoan, Olga, Robert, Sebastien and Eugene on our new paper in Chem. Comm. about Mn complexes with N,S-pyridinophane ligand and their reversible dearomatization, and thanks to everyone for your hard work! Good start of year 2019!

Summer interns at CCCU

We were lucky to host three great undergraduate research interns this summer, Luke (Harvard University), Saiyyna (Lomonosov Moscow State University) and Jiratheep (University of Oxford), who, unfortunately, have to leave our group to continue their studies. Thank you all for your great job and good luck with your future careers!
Pradnya's article is now online!
Our article in Inorganic Chemistry has just appeared online. Congratulations to everyone and thanks for hard work!

Congratulations to Orestes on winning CSJ Presentation Award 2018!
After giving the excellent presentation "Unsymmetrical naphthyridinone based ligand scaffolds for the development of linear chain multimetallic complexes" (4A7-11) at the 98th Annual CSJ meeting, Orestes received CSJ Presentation Award 2018.
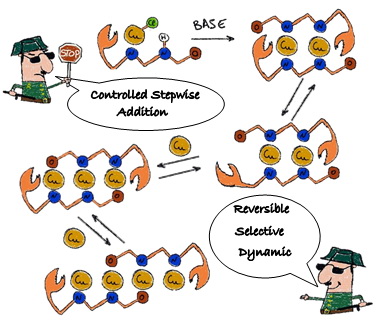
Congratulations to Orestes, Sandra and Robert with ACIE paper!
Congratulations to everyone with our paper "Controlled and reversible stepwise growth of copper(I) linear chains enabled via dynamic ligand scaffolds" that has just been accepted for publication in Angew. Chem. Int. Ed.!
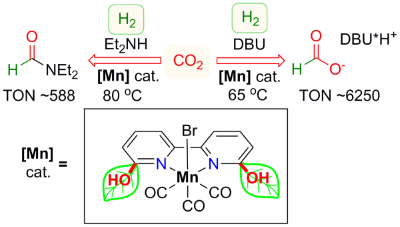
Abhi's paper on manganese-catalyzed CO2 hydrogenation is now published
Congratulations to Abhi, our former intern Luca and Robert (visiting scientist at CCCU), as well the rest of our team!
The article is now highlighted in OIST News: "Recruiting Manganese to Upgrade Carbon Dioxide".
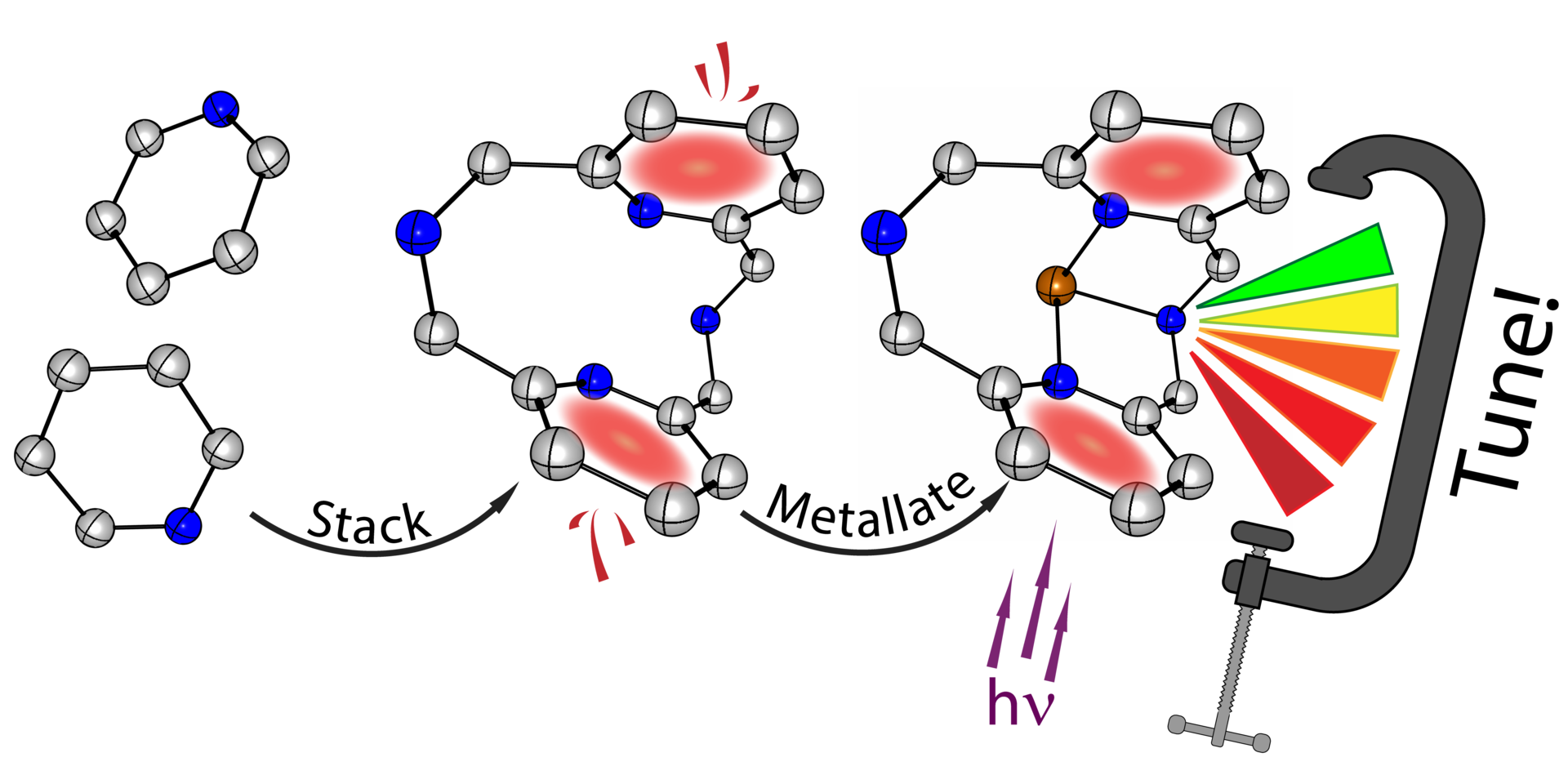
Our Advanced Materials article is in press
Our article in "Advanced Materials" is now in press. It was also highlighted in OIST News: "Twist and Shine: Development of a New Photoluminescent Sensor Material".

Georgy's paper in the news!
Georgy's first paper was recently highlighted in OIST News: "Let it glow". Congratulations!
Course highlights: "Inorganic Electrochemistry: Fundamentals and Applications in Renewable Energy Catalysis"
Now that the class is almost finished (except for the final exam tomorrow), I'll introduce the highlights of my course "Inorganic Electrochemistry: Fundamentals and Applications in Renewable Energy Catalysis". This is a graduate level course for chemistry students that includes both lectures and laboratory experiments. In the class, we introduced basic principles of electrochemistry, and discusses modern research in the application of transition metal complexes in electrocatalysis for renewable energy storage and production. See the highlights of some of the lab experiments and a syllabus below. Read more...
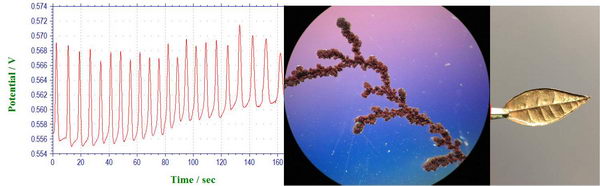
DLA, Electroplating and oscillating reaction lab
Finally, we had our last laboratory experiment in “Inorganic Electrochemistry” class, during which we collected some entertaining experiments for the end of the year: oscillating reactions, electroplating, and diffusion-limited aggregation. Read more...
Electrochemiluminescence and Chemiluminescence Lab
With some delay, updates from the last labs in “Inorganic Electrochemistry” class that I teach this semester. In the lecture, we covered briefly electrochemiluminescence and chemiluminescence, so in the following lab, we tried to see both, ECL by following experiments from Bard’s papers, and for chemiluminescence, just playing with luminol and different catalysts. Almost entire lab was done in the dark. Luckily, we can shut the windows in the teaching lab (luxury we can’t afford in most of the new research labs full of glass walls…) Read more...

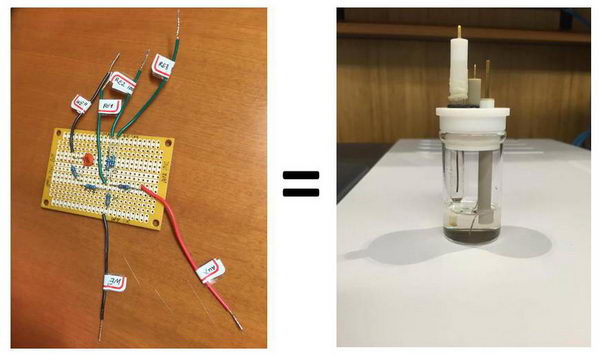
“Wet” and “dry” electrochemistry: playing with equivalent circuit
We just had our first laboratory in the advanced electrochemistry course that I teach this semester, and before starting “wet” electrochemistry in solution, I wanted to do a quick demonstration of the basic electrochemistry experiments using a so-called “equivalent circuit” (“equivalent cell”). I find that it is a nice way to show some of the concepts (and for us, it is also useful to troubleshoot any technical problems with the potentiostat). You could say it is a useless experiment, but it is also a nice way to look at how electrochemistry in solution can be modeled to a certain extent by using a simple circuit. Read more...
Research Interns at CCCU
Welcome to Sandra, our new research intern in the group!
And good luck to our interns who just finished their internship programs, Luca Nencini and Shubham Deolka.

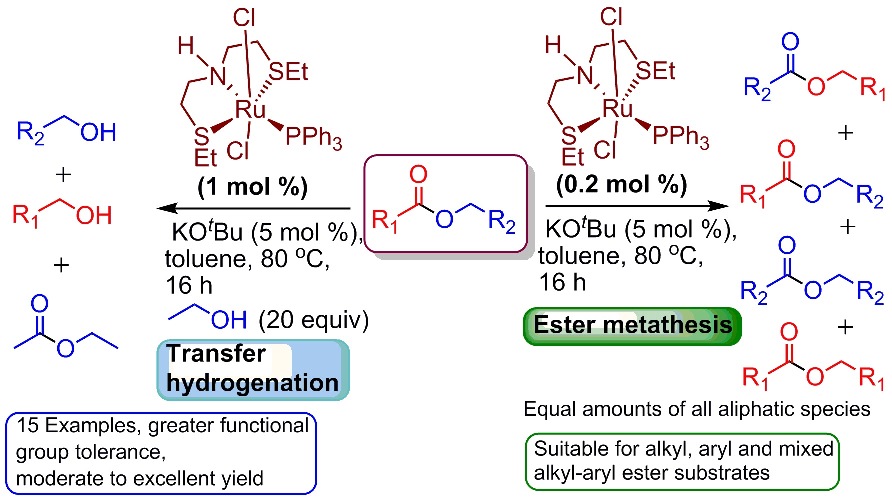
Eugene's and Abhi's first paper
Congratulations to Eugene and Abhi who published their first OIST paper in ACS Catalysis about ester metathesis!
To be presented also at the upcoming ISHC XX meeting in Kyoto.
Investigation of noise
ゴールデンウィーク posting continues. As promised a few months ago, I’m going to talk about some artefacts I’ve found while using an ESR electrolysis cell. This has no practical meaning at all and is meant only as a futile intellectual exercise into what kind of noises you can encounter while doing electrochemistry. Read more...
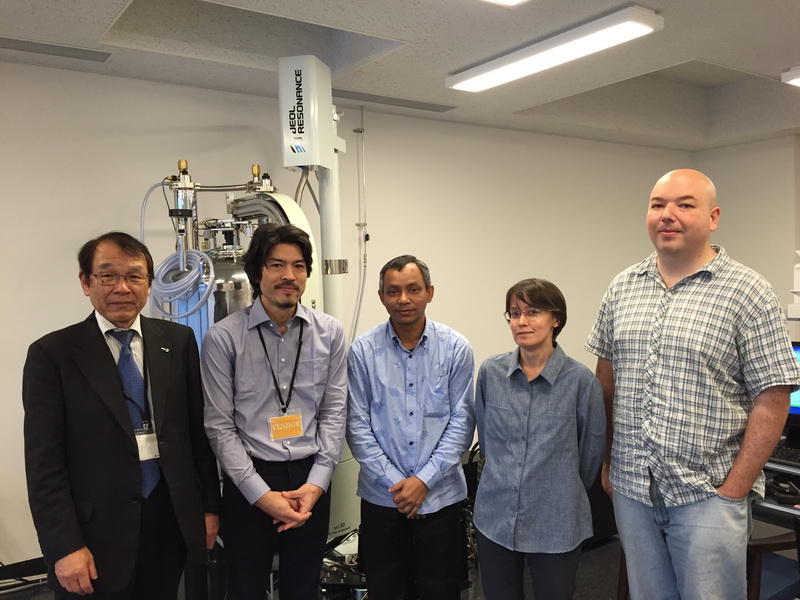
Solid state NMR
Although solid NMR training at OIST finished a couple of weeks ago, I did not have time to write a summary until now. From left to right, training participants and organizers are: Yunhui (Zhang group), Dr. Jun Ashida (JEOL), Eugene Khaskin (OIST), JEOL 600 MHz NMR, Julia, Dr. Koji Yazawa (JEOL), and Michael Roy (OIST).

See below the report on basic solid NMR training.
600 MHz installation
Installation of the common use 600 MHz NMR was finished, thanks to Nagashima-san, who came to Okinawa for the second time after installing the 400 MHz a few months ago. The new instrument can do both solid and liquid NMR and it only takes a few minutes to exchange probes. With a liquid samples probe, it's also possible to measure down to -40C without liquid nitrogen and up to +150C (with air stream cooling to protect against overheating). Long-time variable temperature experiments are thus possible. Read below some highlights from preliminary training...

ESR (EPR) installation
Here is the picture from the last day of ESR installation:
Left to right: Mizuta-san (JEOL), Julia, Eugene (at the back), Roy-san.
Kanai-san from JEOL is not in the picture, since he left one day earlier.
I am holding an electrolysis ESR cell and looking happy because I know I’m going to spend next several days doing something with it that has absolutely no practical meaning (but I will talk about it briefly at the end of this post and leave the rest for the next one). Read more...

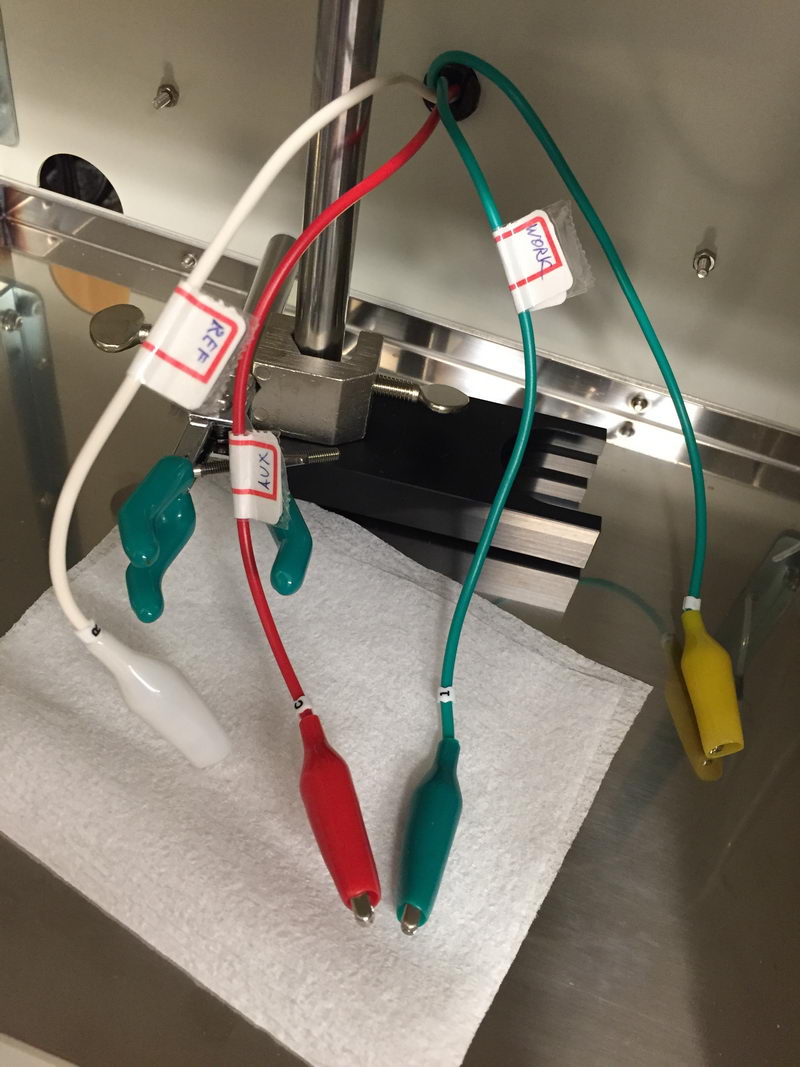
"Cut the red one!!!"
There is sometimes a question when people start doing CV measurements about cable connections: which cable to connect to which electrode? So we have a green one, a red one, and a white one. To give a final reference point for CV experiments and solve this question once and for all, let’s have a look again at the correct way of connecting cables to the electrodes in the three-electrode cell for CV experiments. And I’ll also show what happens if you do it in the wrong way.
What we have in the CV cell is (a) a reference electrode; (b) working electrode (Pt or glassy carbon disk electrode), and (c) auxiliary electrode, usually Pt wire. All our cables are labeled as follows:
Our new arrival...
...weighs 2.3 tonnes.
This is equivalent to an average weight of an adult rhinoceros or a humpback whale.
Setting up the lab: some progress since June 2015 to January 2016, in pictures
Just to document some changes, this is how the lab looks now, and how it was back in June 2015 when we just started. There are definitely some changes that happened over the last half a year, although the process is still in progress.
Thanks to everyone in our unit (Orestes, Eugene, Georgy, Abhi and Chika) and in OIST Facilities Section (Takada-san, Sakurai-san, John Dickison) and to OIST start-up budget for helping set up a working synthetic organometallic lab in a reasonable period of time, and Happy New year!
Synthetic Lab C704 in January 2016:

Same lab in June 2015 when we just started:
JEOL 400 MHz NMR application training
Our first applications training at OIST for JEOL 400 MHz was recently finished.
From left to right: Eguchi-san (JEOL), Pavlos-san (JEOL), Roy, Julia and Eugene (OIST).
Thanks to Pavlos-san, we covered a lot of topics over the past two and a half days of intense training, including: basic 1D experiments, array experiments, basic 2D experiments including data collection and processing in Delta (COSY, NOESY), 1H-15N HMBC, 1D NOE, DOSY, extremely nice No-D experiment (proton in non-deuterated solvents with solvent suppression, eventually it worked perfectly well for our toluene and THF samples), t1 measurements, 90 deg pulse calibration, high temperature kinetics experiments, and we even quickly looked at Saturation-Transfer Difference at the end. Most surprisingly, the autosampler was actually able to handle 7-inch J Young tubes (just don’t try to put longer tubes!).
Preparing for electrochemical experiments inside the glovebox

Since we started getting some really air- and moisture-sensitive complexes, I finally set up the system to use our potentiostat for voltammetric or electrolysis experiments inside the glovebox. There are usually several options how to connect a cable with an electrochemical cell located inside the glovebox (BNC, just hermetically sealed cable feedthrough etc), but we decided to go with simple banana connectors as the most affordable and simple option.
This is the final setup, and below is the story of the whole process in pictures, just in case someone else can find it useful.
Installation of new JEOL 400 MHz NMR is finally finished!
Julia and Nagashima-san
Thanks to JEOL’s Nagashima-san for installation!
What’s nice about OIST is that you get to see installation of all brand new equipment from the very beginning: 
Installation and training for elemental analyzer
Installation of CE440 with Dr. Paul Brockman (Exeter Analytical)

Vacant positions are available!
Applicants for a PhD program can apply directly from OIST Graduate School Admissions website:
Postdoctoral positions are currently available only via application for external fellowship (JSPS).