Annual Report FY2015: Eugene Khaskin
Eugene Khaskin
Science and Technology Associate
1. Introduction
During FY2015 I moved to OIST full time in May, from the Weizmann Institute. In the same month, work that was carried out in the Weizmann laboratories on the olefination of alcohols, while I was on an associated OIST contract during the previous eight months was published in an RSC journal Chemical Communications. This same research was presented as an oral report at the XIIth European Congress on Catalysis in Kazan, Russia in September. The first year at OIST was spent on building up the lab and starting synthesis on my two main projects. The catalysis project (rather rapidly) gave significant results that were written up and submitted for publication right before the end of the fiscal year.
2. Activities and Findings
The laboratory at OIST proved to be easier to set up than planned, as it normally takes a year to set up a fully functional chemistry laboratory. For this the technical staff at OIST have to be commended. I started work on two main projects that were outlined in my proposal already at the end of the summer.
I) Synthesis of modified pincer ligands that can potentially form bimetallic species. A number of new ligands has been made and in some cases complexes have been synthesized (Figure 1).
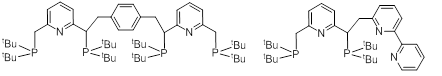
Although the ligands in Figure 1 have been made, and complexes with nickel were realized, including a crystal structure for one of them, during synthesis it was discovered the iPr moieties give much higher yields and result in cleaner complexes. In the future, all synthesis will have to be carried out with iPr instead of tBu moieties. When large amounts of complex become available, synthesis of heterobimetallic complexes will be prioritized.
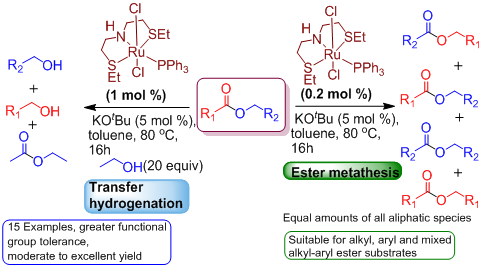
II) Work on the catalysis project result in the significant finding of two new reactions that include ester metathesis (statistical scrambling of unsymmetrical esters) and transfer hydrogenation of esters using primary alcohols as the hydrogen source. Practical transfer hydrogenation of esters that avoids high pressures of explosive H2 gas was thought to be impossible until the current results. Figure 2 is a slightly modified form of the graphical abstract that was submitted for publication before the end of the fiscal year.
3. Collaborations
None to report in this FY.
4. Publications and other output
Author list, Title, Journal or other reference, volume information (year)
Publication
Khaskin, E.; Milstein, D., “Catalytic, oxidant-free, direct olefination of alcohols using Wittig reagents”, Chemical Communications, 2015, 51(43), 9002-9005
Presentation:
Khaskin, E. (presenting author), Milstein, D. “Oxidant-free formal oxidation of alcohol substrates towards higher value added products” oral presentation at the 12th European Congress on catalysis, 30 August – 4 September, 2015, Kazan, Russia.



