Controlled Drugs: Narcotics, Psychotropics, and Stimulants
 Narcotics, psychotropics, and stimulants are indispensable in medicine, education, and academics. Narcotics and psychotropics are essential because of their effects on cognition, sleep-induction, analgesia, etc. Stimulants are essential for their effects on the treatment of attention deficit disorder, narcolepsy, depression, etc. However, narcotics, psychotropic drugs, and stimulants, due to their impacting effects and great potential for dependence, not only harm one’s health when abused but also cause many social problems.
Narcotics, psychotropics, and stimulants are indispensable in medicine, education, and academics. Narcotics and psychotropics are essential because of their effects on cognition, sleep-induction, analgesia, etc. Stimulants are essential for their effects on the treatment of attention deficit disorder, narcolepsy, depression, etc. However, narcotics, psychotropic drugs, and stimulants, due to their impacting effects and great potential for dependence, not only harm one’s health when abused but also cause many social problems.
In Japan, handling of such chemicals is severely restricted to medical or academic purposes only. Management, including acquisition, use, storage, and disposal, is strictly regulated by relevant laws, such as the "Narcotics and Psychotropics Control Act" and the "Stimulants Control Act".
OIST defines narcotics, psychotropics, and stimulants as “Controlled Drugs", and has established the "OIST Rules for Management of Narcotics, Psychotropics and Stimulants" which describes management system, and the use and management of Controlled Drugs. Researchers who use narcotics, psychotropics, or stimulants must understand proper handling procedures (acquisition, storage, disposal, etc.)
Examples at OIST:
- Narcotics: Ketamine hydrochloride (Ketalar)
- Stimulants: Methamphetamine hydrochloride (Philopon)
- Psychotropics: Pentobarbital sodium (Somnopentyl)
Summary
- Narcotics Researcher & Stimulants Researcher
- Narcotics Researcher License / Stimulants Researcher Designation Certificate
- Expiry Date of the License/Designation Certificate
- Notifications of Change, Return, & Discontinuation
- Acquisition of Narcotics and Stimulants
- Storage of Narcotics and Stimulants
- Handling of Narcotics and Stimulants
- Recording
- Disposal and Incidents
- Annual Report
- Periodic Inspection
- Forms for Narcotics research
Prohibitions on Controlled Drugs
- Storing Controlled Drugs in a place other than the designated storage cabinet
- Relocating Controlled Drugs to a place outside of the OIST main campus. Note that the Okinawa Marine Science Station has a different address and thus is considered outside of the OIST main campus
- Transfer-in or -out of narcotics or stimulants from/to an unauthorized researcher regardless of whether it takes place on the premises of OIST or in other locations
- Transfer-in or -out of any narcotics or stimulants (including those after subdivision or dilution)
- Use of Controlled Drugs for purposes other than for education or academic research
- Synthesis or manufacture
- Import or export
Narcotics Researcher & Stimulants Researcher
Narcotics and stimulants are strictly regulated, even for educational or academic research purposes. Compliance with rigorous management protocols and submission of various notifications are mandatory.
Please refer to online training "Safety Training on Narcotics, Psychotropics, and Stimulants".
Narcotics Researcher License / Stimulants Researcher Designation Certificate
It is necessary to apply for a narcotics researcher license or stimulant researcher designation certificate to handle narcotics or stimulants, respectively, for education or academic research.
It takes about 1 to 2 months to obtain the permit.
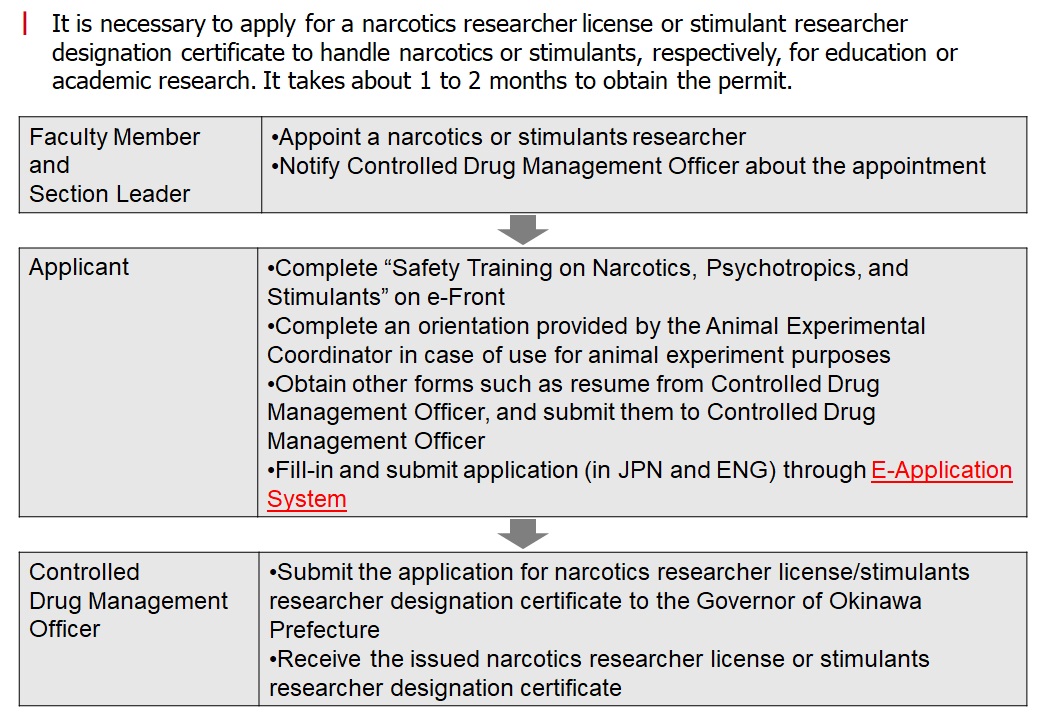
Procedures for narcotics researcher license/stimulants researcher designation certificate
Expiry Date of the License/Designation Certificate
Narcotics researcher license is valid until Dec. 31 of the third year from the issued year.
Stimulant researcher designation certificate is valid until Dec. 31 of the second year from the issued year.
Notifications of Change, Return, & Discontinuation
If there are any changes in the license/designation certificate or if you no longer need the license/designation certificate, the researcher must submit the notification to the governor within15 days of the change/expiration/discontinuation.

Acquisition of Narcotics and Stimulants
After obtaining a permit of purchase from the Controlled Drug Management Officer, narcotics and stimulants can only be purchased from a commercial narcotics handler and a stimulants manufacturer, respectively.
Application for permission purchase narcotics/stimulants (web form)
Acquisition of narcotics/stimulants
Storage of Narcotics and Stimulants
Narcotics and stimulants (including those after subdivision or dilution) must be stored in the narcotics/stimulants storage cabinets specified in the application. Narcotics and Stimulants can be stored in the same storage cabinet, other items including psychotropics, other reagents, and logbooks shall not be stored with them.
- Apply measures to a container of narcotics/stimulants to prevent evaporation of the solvent, e.g., applying a parafilm on the lid of the container
- A "storage" is a robust cabinet fixed to the wall/floor and double-locked at all times
- A new storage cabinet needs to be inspected by the Okinawa Governor's officials after applying for a license/designation certificate.
- The key to the storage cabinet must be securely managed, e.g., keeping the key in an electronic or pin code key box.
Handling of Narcotics and Stimulants
Handling of narcotics and stimulants is strictly regulated.
- Never use narcotics/stimulants for a purpose other than that described in the application
- Always keep narcotics/stimulants within your sight after taking them out of the storage cabinet and while working with them
- Understanding the properties of narcotics/stimulants well before using them
- Regularly check the remaining amount to prevent loss by evaporation of solvents or theft
Wear appropriate PPE to prevent exposure to narcotics or stimulants. Never recap a needle when use a syringe.
It is essential to keep a strict record of the usage of narcotics and stimulants.
You must make your own log sheet for each container upon delivery, subdivision, or dilution of narcotics/stimulants. You must also make a record of all transfers including the amount of use, dilution, and adjustment, in the log sheet.
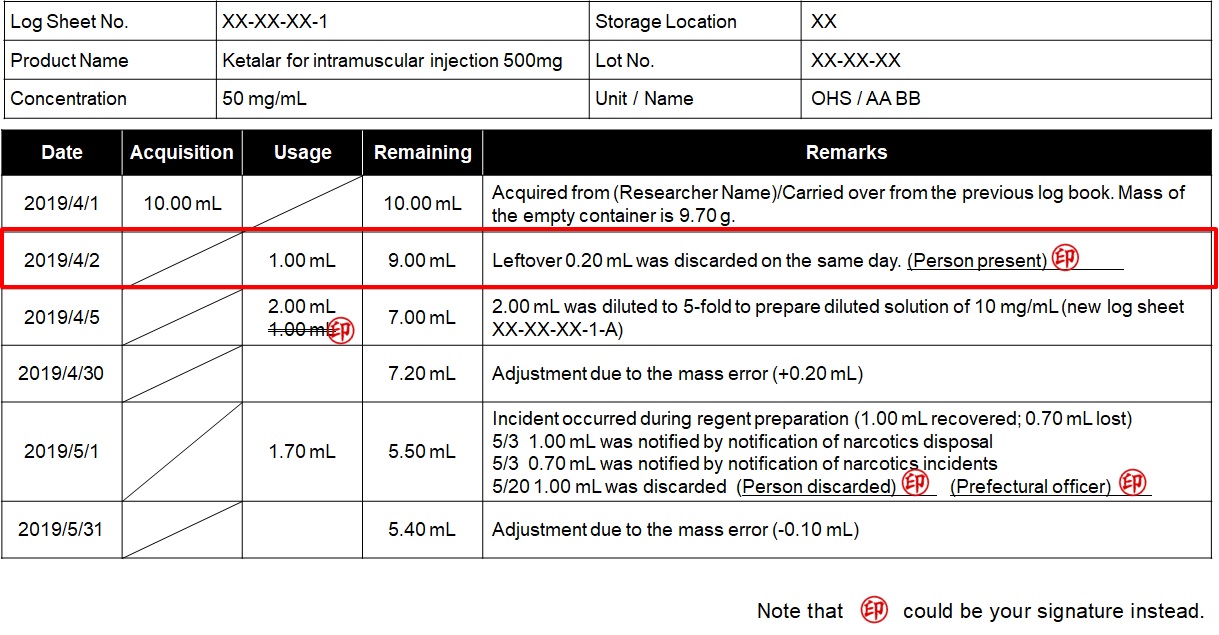
Important points to keep in mind when making entries in the logbook.
- Every form of use, including subdivision, dilution, etc., of narcotics and stimulants shall be recorded in black pen in their respective logbook.
- Record the weight of the empty container and the weight of the container filled with narcotics or stimulants, and accurately record the weight after every use, dilution, and subdivision.
- To ensure accurancy in the recorded amounts of narcotics and stimulants during periodic and annual inspections, conduct regular verification of the remaining amount (weight) at least once a month. If discrepancies are found between the recorded and the actual amount (weight), record the weighing error in the remarks column of the logbook.
- When you make a mistake in the logbook, make a double-line strikethorough the mistake entry, and stamp it with your seal or sign it.
- The volume disposed of and lost in accidents shall be entered in the remarks column.
For disposal of unnecessary narcotics/stimulants, submit a notification of disposal under the name of the OIST's President, and dispose of them by a method that prevents recollection in the attendance of an official(s) of the Okinawa Prefectural Government. Do not dispose of without permission.
In case of any loss, theft, or missing of narcotics/stimulants, immediately report to your supervisor and submit a notification of incidents to the Okinawa Prefectural Government via the Controlled Drug Management Officer.
Each narcotics researcher must summarize all records from Oct. 1 to Sept. 30, and submit an annual report to the Governor through the Controlled Drug Management Officer by Oct. 31.
Each stimulant researcher must summarize all records from Dec. 1 to Nov. 30, and submit an annual report to the Governor through the Controlled Drug Management Officer by Dec. 5.
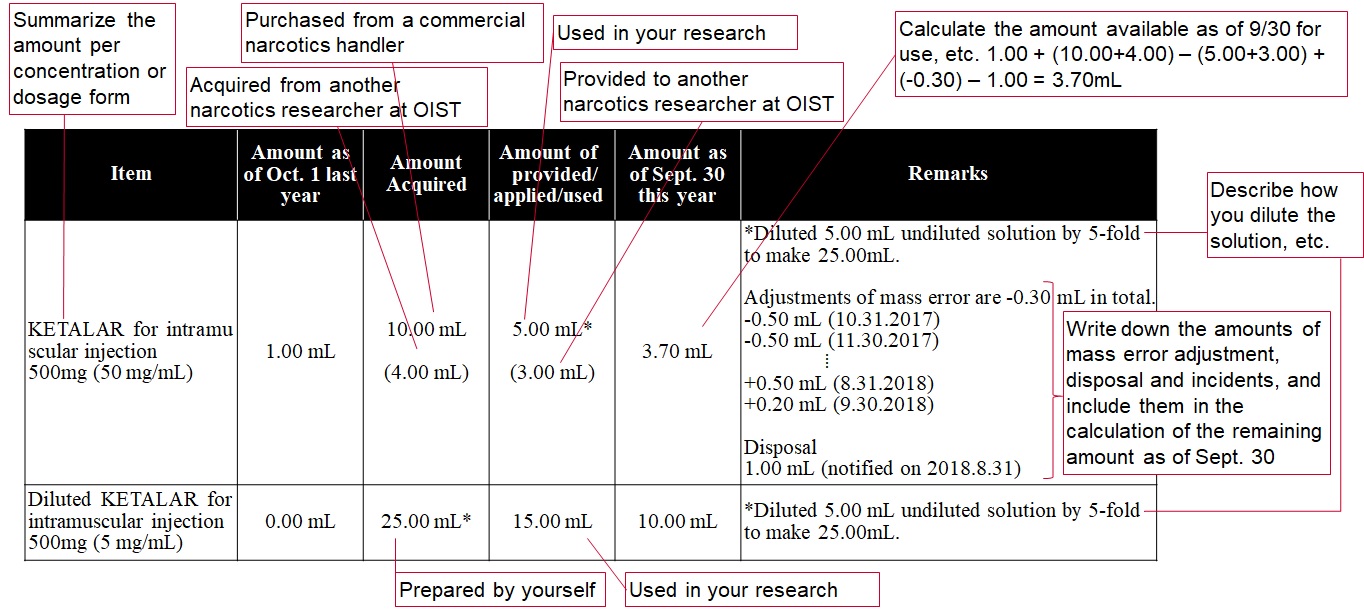
An annual On-Site Inspection will be performed to confirm the contents of the annual report.
Narcotics and stimulants researcher must not only periodically check their controlled substances, but also perform the stocktake once a year and report the results to the Controlled Drug Manager. The Controlled Drug Manager may conduct an inventory inspection without prior notice.
- Application for license (application form, medical certificate, CV) [word, PDF]
- Notification of change [word]
- Notification of return [word]
- Notification of discontinuation [word]
- Notification of disposal [word]
- Notification of incident [word]
- Annual report [word]
- Logsheet for Narcotics [word]
Psychotropics Researcher
Psychotropics are strictly regulated even for education or academic research. It is mandatory to comply with rigorous management protocols and to submit various notifications.
Please refer to online training "Safety Training on Narcotics, Psychotropics, and Stimulants".
Appointment of Psychotropics Researcher
In priciple synthesis or manufacture of Controlled Drugs is prohibited, however, you can handle psychotropics for education or academic research after completing the following internal procedures.
Procedures for appointment of psychotropics researcher
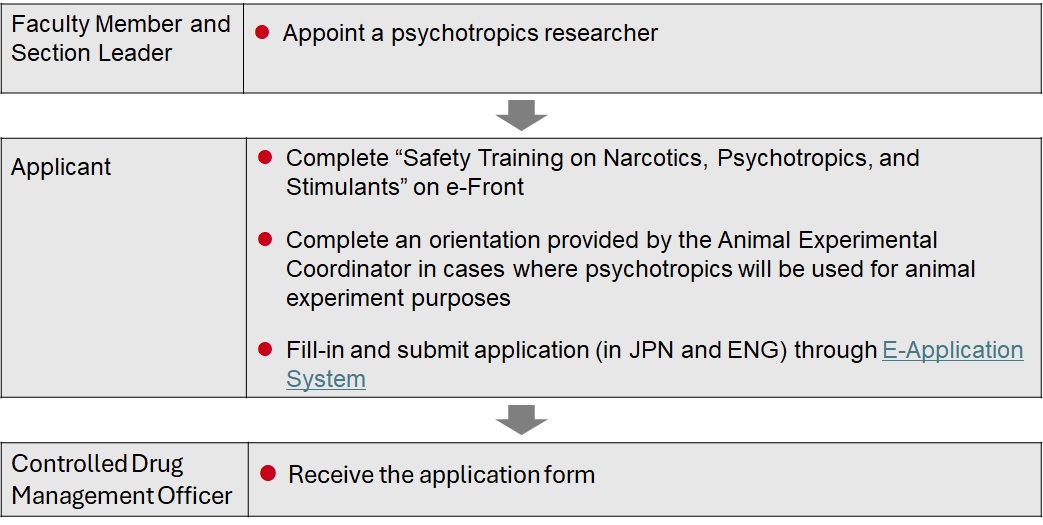
Acquisition
It it necessary to obtain a permit of pruchase for purchasing Type1 and Type 2 Psychotropics. After obtaining a permit of purchase from the Controlled Drug Management Officer, psychotropics can only be purchased from a manufacturer, formulator, or wholesaler having a psychotropics license, or another registered psychotropic academic research facility.
Application for permission to purchase psychotropics(web form)
Storage
All psychotropics (including those after subdivision and dilution) must be stored in a locked storage cabinet. Never store psychotropics in the storage cabinet for narcotics or stimulants.
Handling
Recording
You must make a log sheet for each container upon delivery, subdivision of psychotropics. You must also make a record of all acquisition, accident, loss, disposal and transfer-out, transfer-in in the log sheet. You can register psychotropics in Chemical Management System (CMS) and record the usage and disposal as a log sheet.
Disposal and Incidents
You can dispose of psychotropics by yourself using a method that makes it difficult to recover them. There is no obligation to submit a notification.
Immediately submit a notification of incidents to the prefecture in case of any loss, theft, or missing of psychotropics that you have.
Forms for Psychotropics
- Logsheet for psychotropics [word]
Periodic Inspection
Controlled drug researchers are not only required to conduct regular self-check of the psychotropics that they manage, but are also required to conduct the annual stocktake and report the results to the Controlled Drug Management Officer. The Controlled Drug Management Officer may conduct inspections without any advance notice.



