FY2015 Annual Report
Energy Materials and Surface Sciences Unit

Abstract
Currently, crystalline silicon solar cell is still dominating the photovoltaic market. The highest solar energy-to-electricity conversion efficiency for crystalline Si cells is around ~25%, but the process to fabricate crystalline Si cells is energy-intensive, which makes the overall cost significantly higher than conventional energy supply (e.g. fossil fuels) and payback time very long (typically > 10 years). On the other hand, perovskite solar cells have recently emerged as a promising candidate for the next generation high efficiency solar cell technology that is compatible with low cost and large-area fabrication. Efficiencies exceeding 20% have been already reported. At the current research stage, however, further improvements are still needed. In particular, development of protocols to make such a technology applicable to industrial-scale is of paramount importance. In addition, lifetime is a major issue and its understanding is largely elusive at present. The third main issue concerns the continuous utilization of electricity generated from this intermittent renewable source (solar energy). Development of efficient electrical energy storage systems (e.g. batteries) will play a vital role in the future use of solar energy. These three aspects constitute the backbone for the research in my Unit.
1. Staff
• Dr. Michael V. Lee, Group Leader
• Dr. Luis K. Ono, Group Leader
• Dr. Min-Cherl Jung, Researcher
• Dr. Gueorgui O. Nikiforov, Researcher
• Dr. Yuichi Kato, Researcher
• Dr. Matthew R. Leyden, Researcher
• Dr. Sonia Ruiz Raga, Researcher
• Dr. Shenghao Wang, Researcher
• Dr. Mikas Remeika, Researcher
• Dr. Robin Ohmann, Researcher
• Dr. Yan Jiang, Researcher
• Dr. Emilio J. Juarez-Perez, Researcher
• Dr. Taehoon Kim, Researcher
• Mr. Zafer Hawash, OIST Graduate Student
• Mr. Collin Chandler Stecker, OIST Rotation student
• Mr. Ankur Dhar, OIST Rotation student
• Mr. Andrew Justin Winchester, OIST Rotation student
• Mr. Atsushi Gabe, Research Intern Student
• Ms. Darya Shchepanovska, Research Intern Student
• Ms. Naoko Ogura Gayler, Research Unit Administrator
2. Collaborations
- Brookite-TiO2 electron transport layer for perovskite solar cells.
- Type of collaboration: collaborative research
- Collaborators:
- Prof. Tsutomu (Tom) Miyasaka, Toin University of Yokohama, Kanagawa, Japan
- Li/S ion battery.
- Type of collaboration: collaborative research
- Collaborators:
- Prof. Yuegang Zhang, i-Lab, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Jiangsu, P.R. China
3. Activities and Findings
3.1 Air Exposure Induced Gas Molecule Incorporation into Spiro-MeOTAD Films.
Yuichi Kato, Luis K. Ono, Michael Vernon Lee, Shenghao Wang, Sonia R. Raga and Yabing Qi*, "Silver iodide formation in methyl ammonium lead iodide perovskite solar cells with silver top electrodes". Adv. Mater. Interfaces 2, 1500195 (2015).
Luis K. Ono+, Sonia R. Raga+, Mikas Remeika, Andrew J. Winchester, Atsushi Gabe and Yabing Qi*, "Pinhole-free hole transport layer significantly improves stability of MAPbI3-based perovskite solar cells under operation conditions". J. Mater. Chem. A 3, 15451-15456 (2015). (+equal contribution)
The stability of solar cells under storage and operation conditions is an important factor in determining the potential for commercial deployment as new photovoltaic technology. Despite all the superb properties of perovskite material, the reported data on the lifetime and degradation mechanisms of fabricated perovskite-based solar cells under operation conditions are limited, although degradation of the perovskite layer induced by ambient air/moisture, temperature and light exposure is evident. However, the degradation mechanism within the device is not well understood. Also, a perovskite solar cell contains several layers, all of which may play a role in the cell stability. For example, little attention has been given to the adjacent electron- and hole-transport layers (ETL and HTL) as well as top-electrode contacts (e.g. Ag, Al, etc.), which might fail faster than the active layer itself. Because in the majority of the perovskite solar cells, the HTL is the top-most layer (i.e. in direct contact with ambient air), the investigation of the physico-chemical property changes induced by air exposure on the HTL is important and need to be investigated systematically (humidity, temperature, and light intensity). 2,2’,7,7’-tetrakis(N,N-di-p-methoxyphenylamine)-9,9’-spirobifluorene (spiro-MeOTAD) is the most widely used HTL in current high performance solid-state cells, mostly due to its high stability (glass-transition temperature Tg = 121 °C), high solubility, and amorphous nature. We have found out that the HTL’s conductivity as well as interfacial energy levels are sensitively affected by the air exposure (O2 and/or H2O). In addition, these as-prepared spin coated films of spiro-MeOTAD showed a high density of pin-holes, which are detrimental for the underneath perovskite layer by facilitating the diffusion of H2O molecules inwards and attacking the perovskite layer.


Figure 1: (Left cover page) Silver is a low-cost electrode material for perovskite solar cells. However, silver electrodes turn yellow after device fabrication, which is accompanied by a decrease in power conversion efficiency. These changes are caused by the formation of silver iodide and iodine-containing compounds from the perovskite layer. The presence of pinholes in spiro-MeOTAD hole transport layer facilitates silver iodide formation. (Right cover page) showcasing the influences of pinholes in hole transport layers on the stability of perovskite solar cells.
3.2 Review Paper on Vapor-Based Techniques for Perovskite Fabrication.
Luis K. Ono+, Matthew R. Leyden+, Shenghao Wang and Yabing Qi*, "Organometal Halide Perovskite Thin Films and Solar Cells by Vapor Deposition" J. Mater. Chem. A 4, 6693-6713 (2016). (+Contributted equally)
Included in the following themed issue: Emerging Investigators 2016: Novel design strategies for new functional materials.
Organometal halide perovskites (OHPs) are currently under the spotlight as promising materials for new generation low-cost, high-efficiency solar cell technology. Within a few years of intensive research, the solar energy-to-electricity power conversion efficiency (PCE) based on OHP materials has rapidly increased to a level that is on par with that of even the best crystalline silicon solar cells. However, there is plenty of room for further improvements. In particular, the development of protocols to make such a technology applicable to industry is of paramount importance. Vapor based methods show particular potential in fabricating uniform semitransparent perovskite films across large areas. In this article, we review the recent progress of OHP thin-film fabrication based on vapor based deposition techniques. We discuss the instrumentation and specific features of each vapor-based method as well as its corresponding device performance. In the outlook, we outline the vapor deposition related topics that warrant further investigation.

Figure 2: Organometal halide perovskite thin films and solar cells by vapor deposition.
3.3 Organic-Inorganic Hybrid Perovskite Atomic Resolution.
R. Ohmann, L. K. Ono, H.-S. Kim, H. Lin, M. V. Lee, Y. Li*, N.-G. Park*, Y. B. Qi*, “Real-Space Imaging of the Atomic Structure of Organic–Inorganic Perovskite”, J. Am. Chem. Soc. 137, 16049 (2015).
(OIST News1, OIST News2, OIST Update - February 2016)
Organic−inorganic perovskite is a promising class of materials for photovoltaic applications and light emitting diodes. However, so far commercialization is still impeded by several drawbacks. Atomic-scale effects have been suggested to be possible causes, but an unequivocal experimental view at the atomic level is missing. Here, we present a low-temperature scanning tunneling microscopy study of single crystal methylammonium lead bromide CH3NH3PbBr3. Topographic images of the in situ cleaved perovskite surface reveal the real-space atomic structure. Compared to the bulk we observe modified arrangements of atoms and molecules on the surface. With the support of density functional theory we explain these by surface reconstruction and a substantial interplay of the orientation of the polar organic cations (CH3NH3)+ with the position of the hosting anions. This leads to structurally and electronically distinct domains with ferroelectric and antiferroelectric character. We further demonstrate local probing of defects, which may also impact device performance.
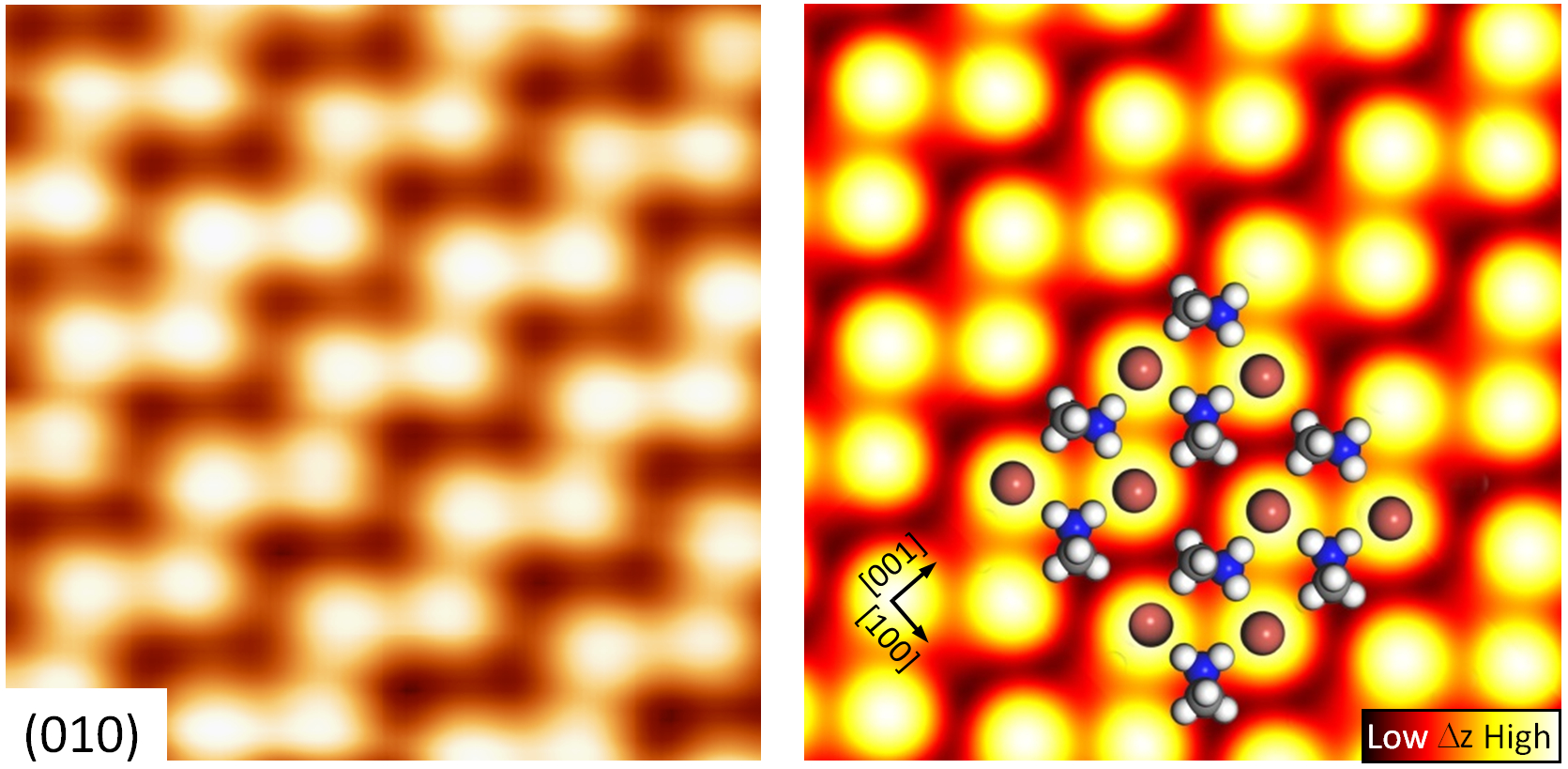
Figure 3: Topography image of atoms of the perovskite crystal and calculated images with position of atoms and molecules indicated.
3.4 Methyl-Amine Gas-Induced Crystallization (MAGIC) for Perovskite Growth.
S. R. Raga, L. K. Ono, Y. B. Qi*, “Rapid Perovskite Formation by CH3NH2 Gas-Induced Intercalation and Reaction of PbI2”, J. Mater. Chem. A 4, 2494-2500 (2016).
Solution processable perovskite solar cells traditionally use CH3NH3I solid powder as one of the two precursors that requires solvation into a solution and a spin-coating step; the resulting films need post- annealing (~1 h) for complete conversion to CH3NH3PbI3. Here we describe for the first time the formation of stoichiometric perovskite in ambient air by exposing PbI2 films to a simple CH3NH2 gas precursor (as opposed to CH3NH3I solid powders). The reaction completes within a few seconds forming complete-coverage perovskite films with a roughness of 2 nm. The non-stoichiometric reaction produces Pb oxides as by-products, which are reconverted by further HI gas exposure. With combined measurements of the thin film crystal structure, chemical state, and absorption properties, we elucidate the chemical reaction mechanisms underlying these gas-induced processes. Fabricated solar cell devices show an efficiency of 15.3%, which remains almost the same after 133 days. Such a gas-induced reaction also enabled the successful preparation of high quality perovskite films with a size of 100 cm2.
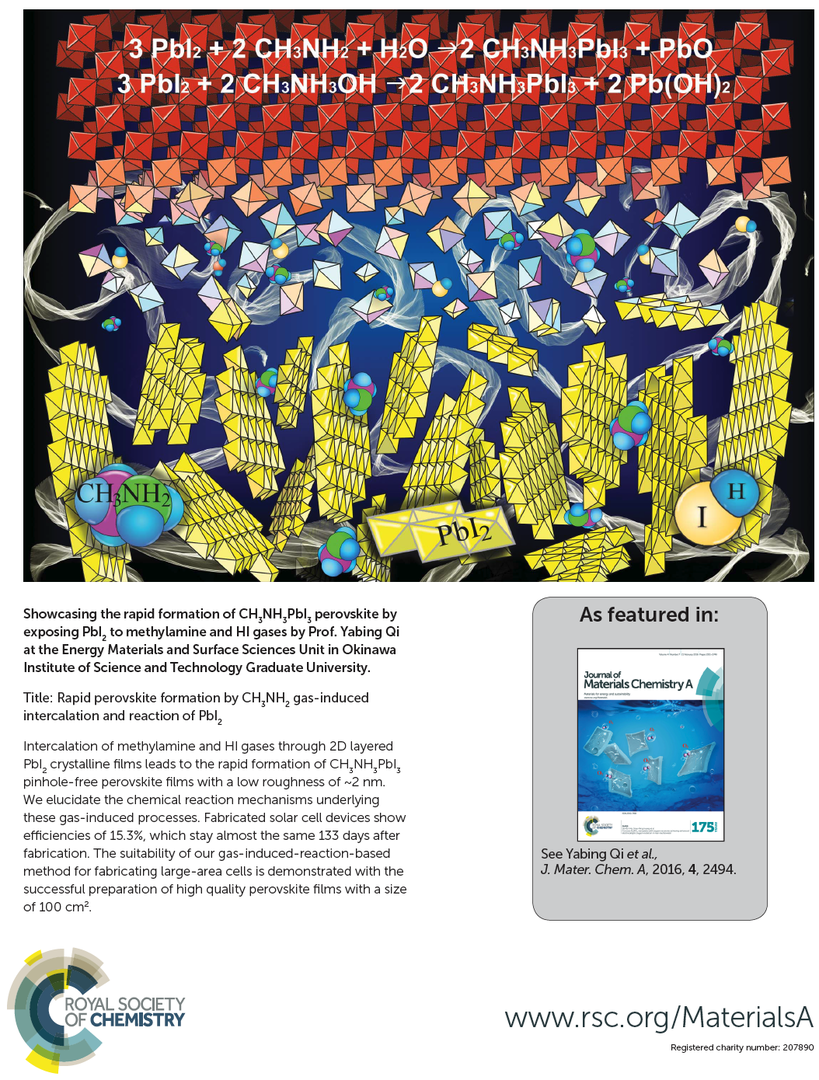
Figure 4: Showcasing the rapid formation of CH3NH3PbI3 perovskite by exposing PbI2 to methylamine and HI gases.
4. Publications
4.1 Journals
- M.-C. Jung, Y.M. Lee, H.K. Lee, J. Park, S.R. Raga, L.K. Ono, S. Wang, M.R. Leyden, B.D. Yu, S. Hong and Y.B. Qi*, "The presence of CH3NH2 neutral species in organometal halide perovskite films" Appl. Phys. Lett. 108, 073901 (2016).
- M.-C. Jung, Y. B. Qi*, "Dopant interdiffusion effects in n-i-p structured spiro-OMeTAD hole transport layer of organometal halide perovskite solar cells", Org. Electron. 31, 71–76 (2016).
- M.-C. Jung, S. R. Raga, Y. B. Qi*, "Properties and solar cell applications of Pb-free perovskite films formed by vapor deposition". RSC Adv. 6, 2819 (2016).
- S. R. Raga, L. K. Ono, Y. B. Qi*, "Rapid Perovskite Formation by CH3NH2 Gas-Induced Intercalation and Reaction of PbI2". J. Mater. Chem. A 4, 2494-2500 (2016).
- K.G. Lim, S. Ahn, Y.H. Kim, Y.B. Qi and T.W. Lee, Universal energy level tailoring of self-organized hole extraction layers in organic solar cells and organic–inorganic hybrid perovskite solar cells. Energy Environ. Sci. 9, 932 (2016).
- R. Ohmann, L. K. Ono, H.-S. Kim, H. Lin, M. V. Lee, Y. Li*, N.-G. Park*, Y. B. Qi*, “Real-Space Imaging of the Atomic Structure of Organic–Inorganic Perovskite”, J. Am. Chem. Soc. 137, 16049 (2015).
- L. K. Ono, S. R. Raga, M. Remeika, A. J. Winchester, A. Gabe and Y. B. Qi*, "Pinhole-free hole transport layer significantly improves stability of MAPbI3-based perovskite solar cells under operation conditions", J. Mater. Chem. A 3, 15451-15456 (2015).
- Y. Kato, L. K. Ono, M. V. Lee, S. Wang, S. R. Raga and Y. B. Qi*, "Silver iodide formation in methyl ammonium lead iodide perovskite solar cells with silver top electrodes". Adv. Mater. Interfaces 2, 1500195 (2015).
- M. R. Leyden, M. V. Lee, S. R. Raga and Y. B. Qi*, "Large formamidinium lead trihalide perovskite solar cells using chemical vapor deposition with high reproducibility and tunable chlorine concentrations". J. Mater. Chem. A 3, 16097-16103 (2015).
- S. Wang, L. K. Ono, M. R. Leyden, Y. Kato, S. R. Raga, M. Vernon Lee and Y. B. Qi*, "Smooth perovskite thin films and efficient perovskite solar cells prepared by the hybrid deposition method". J. Mater. Chem. A 3, 14631-14641 (2015).
4.2 Books and Other One-Time Publications
nothing to report
4.3 Oral and Poster Presentations
- Y.B. Qi, “A Surface Science Approach to Perovskite Solar Cell Research”, 2016 MRS Spring Meeting & Exhibition, Phoenix, USA (March 28- April 1, 2016) (Invited talk)
- Y.B. Qi, “A Surface Science Approach to Perovskite Solar Cell Research”, Hybrid-Photovoltaics 2015 Symposium, Berlin, Germany (December 10-11, 2015) (Invited talk)
- Y.B. Qi, “A Surface Science Approach to Perovskite Solar Cell Research”, Dresden University of Technology, Dresden, Germany (December 8, 2015) (Invited talk)
- Y.B. Qi, “Perovskite solar cells with improved operation stability”, the 7th Asian Conference on Organic Electronics 2015 (A-COE 2015), Beijing, China (October 29-31, 2015) (Invited talk)
- Y.B. Qi, “A Surface Science Approach to Perovskite Solar Cell Research”, Stanford University, Stanford, USA (April 3, 2015) (Invited talk)
- Y.B. Qi, “A Surface Science Approach to Perovskite Solar Cell Research”, Princeton University, Princeton, USA (April 1, 2015) (Invited talk)
- M.R. Leyden, L.K. Ono, Sonia R. Raga, Yuichi Kato, Shenghao Wang, and Yabing Qi*, “High Performance Perovskite Solar Cells by Hybrid Chemical Vapor Deposition.”, 2015 MRS Spring Meeting & Exhibit, San Francisco, California, April 6-10, 2015.
- L.K. Ono, Sonia R. Raga, Shenghao Wang, Yuichi Kato, and Yabing Qi*, “Temperature-dependent hysteresis effects on perovskite-based solar cells.”, 2015 MRS Spring Meeting & Exhibit, San Francisco, California, April 6-10, 2015.
- L.K. Ono and Yabing Qi*, “Stability issues in perovskite-based solar cells: Considerations beyond efficiency.”, OIST Internal Seminar, Japan, June 26, 2015.
5. Intellectual Property Rights and Other Specific Achievements
- Yabing Qi, Sonia Ruiz-Raga, Luis K. Ono, US provisional patent application (2015).
- Yabing Qi, Sonia Ruiz-Raga, US provisional patent application (2015).
- Yabing Qi, Min-Cherl Jung, Sonia Ruiz-Raga, US provisional patent application (2015).
6. Meetings and Events
6.1 Seminar
- Date: April 13, 2015
- Venue: OIST Campus Lab1, C016
- Speaker: Prof. Zhengtao Xu, Department of Biology and Chemistry, City University of Hong Kong, Hong Kong
- Title: Sulfur empowers porous frameworks: sensors, catalysts and a story of bridgin the gaps.
- Date: April 22, 2015
- Venue: OIST Campus Lab1, C016
- Speaker: Prof. Shuzi Hayase, Graduate School of Life Science and Systems Engineering, Kyushu Institute of Technology, Kitakyushu, Japan
- Title: Printable Solar Cells.
- Date: April 22, 2015
- Venue: OIST Campus Lab1, C016
- Speaker: Prof. Tingli Ma, Graduate School of Life Science and Systems Engineering, Kyushu Institute of Technology, Kitakyushu, Japan
- Title: Development of nano functional materials and their application in organic/inorganic hybrid solar cells.
- Date: April 30, 2015
- Venue: OIST Campus Lab1, C016
- Speaker: Prof. Sang-Wook Han, Jeonbuk National University, Korea
- Title: Introduction of XAFS adn its applications.
- Date: May 7, 2015
- Venue: OIST Campus Lab1, C016
- Speaker: Prof. Yuegang Zhang, i-Lab, Suzhou Institute of Nano-Tech and Nano- Bionics, Chinese Academy of Sciences, Jiangsu, P.R. China
- Title: Chemical Routes toward Long-Lasting Lithium/Sulfur Cells.
- Date: June 6, 2015
- Venue: OIST Campus Lab1, C016
- Speaker: Prof. Tsutomu (Tom) Miyasaka, Toin University of Yokohama, Kanagawa, Japan
- Title: Organo lead halide perovskite for high efficiency printable solar cells and optoelectronic devices.
- Date: August 9, 2015
- Venue: OIST Campus Lab1, C016
- Speaker: Dr. Byung-wook Park, Konkuk University, South Korea
- Title: To Understand Effect of Order-Disorder Phase Transitions Within Hybrid Perovskiet Ligtht Absorbers and Solar Cell Application.
- Date: December 15, 2015
- Venue: OIST Campus Lab1, C016
- Speaker: Prof. Susumu Yanagisawa, Department of Physics and Earth Sceinces, Faculty of Science, University of the Ryukyus, Okinawa, Japan
- Title: First-principles investigation on the electronic states of the organic single-crystals and the organic-metal interfaces: importance of accurate prediction of the crystal and the interface structures.



