FY2020 Annual Report
G0 Cell Unit
Professor Mitsuhiro Yanagida

Abstract
The G0 Cell Unit studies how cells in the proliferative (dividing) or quiescent (non-dividing) phase respond to nutritional shifts (e.g., nitrogen source starvation and glucose limitation). We identify gene products (mostly proteins) and chemical factors (small molecules, metabolites) that affect adaptation mechanisms in order to understand the basis for longevity of long-term quiescent cells. We employ fission yeast as a eukaryotic cell model as the great majority of this organism’s genes are conserved in human. In addition, we are studying chromosomal regulatory mechanisms involving condensin, cohesin complexes (which lead to proper chromosome segregation in proliferating cells), and other nutrient adaptation-related nuclear chromatin proteins. This line of studies aims to understand dynamics of nuclear chromatin in response to nutritional cues. Study on human blood metabolomics initiated from comparative study with fission yeast metabolomics now directly aims to identify and understand the roles of metabolites intimately related to human aging.
Our current principal research projects may be summarized as below:
(1) Metabolomic approach to human aging and aging-related diseases.
(2) Understanding cell regulation in response to nitrogen deprivation.
(3) Understanding cell regulation in response to glucose starvation.
(4) Understanding chromosomal regulatory mechanisms involving condensin, cohesin complexes, and other nutrient adaptation-related nuclear proteins.
In FY2020, we published 6 original articles on research topics including chromosome regulatory mechanisms, genetic regulation for nutritional response, and human metabolomic analysis and filed 3 patents, as listed in 3. Activities and Findings, 4. Publications, and 5. Intellectual Property Rights and Other Specific Achievements.
1. Staff
- Dr. Xingya Xu, Staff Scientist
- Dr. Michiko Suma, Postdoctoral Scholar
- Dr. Yunfui Zheng, Postdoctoral Scholar (until July, 2020)
- Ms. Orie Arakawa, Technician
- Ms. Ayaka Mori, Technician
- Ms. Yuria Tahara, Technician
- Dr. Takayuki Teruya, Technician
- Ms. Risa Uehara, Technician
- Ms. Li Wang, Technician
- Ms. Chikako Sugiyama, Research Unit Administrator
2. Collaborations
- Theme: Analysis of human blood metabolites, involved in aging and aging-related diseases
- Type of collaboration: Joint research
- Researchers:
- Dr. Hiroshi Kondoh, Department of Geriatric Medicine, Graduate School of Medicine, Kyoto University
- Dr. Takumi Mikawa, Department of Geriatric Medicine, Graduate School of Medicine, Kyoto University
- Dr. Masahiro Kameda, Department of Geriatric Medicine, Graduate School of Medicine, Kyoto University
- Theme: Comparative analysis of blood metabolome between healthy people and metabolic abnormality patients
- Type of collaboration: Joint research
- Researchers:
- Professor Hiroaki Masuzaki, Division of Endocrinology, Diabetes and Metabolism, Hematology, Rheumatology (Second Department of Internal Medicine), Graduate School of Medicine, University of the Ryukyus
- Theme: Blood metabolome analysis in abnormal cerebral function
- Type of collaboration: Joint research
- Researchers:
- Dr. Yasuhide Fukuji, Director, National Hospital Organization Ryukyu Hospital
- Dr. Taku Otsuru, Deputy Director, National Hospital Organization Ryukyu Hospital
3. Activities and Findings
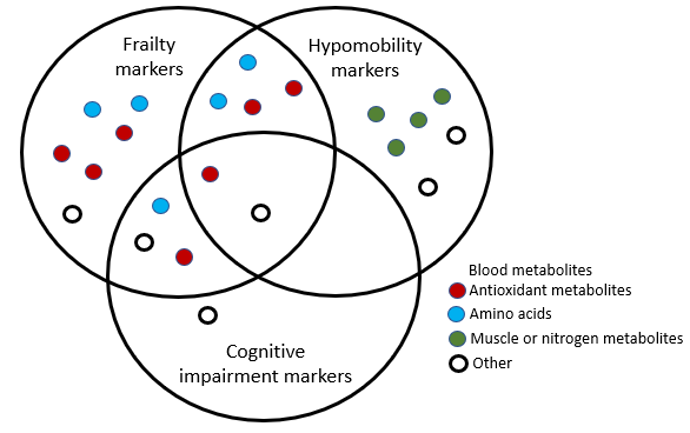
3.1 Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility (From OIST news center article "Leaving its mark: How frailty impacts the blood" by Lucy Dickie)
Globally, human society is aging. A side-effect of this is that age-related disorders, such as frailty, are becoming increasingly common. Frailty includes, not only physical disabilities, but also a decline in cognitive function and an increase in various social problems. The prevalence of this disorder among those aged 65 and over is estimated at 120 million people worldwide.
But, due to their small range of activities, people who suffer from frailty are often hidden. They tend to stay at home and out of the public eye. They can struggle to walk, suffer from memory loss, and find essential tasks, like putting out the rubbish or cleaning the house, very difficult. As such, frail people require more help than their healthy peers. And although there has been some indication that frailty may be reversible, no such interventions have yet been established.
The first step to curing frailty is to find an efficient way to diagnose the disorder. Researchers from the G0 Cell Unit at the Okinawa Institute of Science and Technology Graduate University (OIST), alongside collaborators at the Geriatric Unit at Kyoto University have taken a close look at the blood metabolites of both frail and non-frail elderly patients using a technique called metabolomics. They’ve found 15 metabolites whose levels in the blood correlate with frailty. Their findings, published in PNAS, have shed light on what causes the disorder and how we might reverse it.
Measuring frailty
For this study, the researchers looked at 19 elderly patients, all above the age of 75, and measured whether they suffered from frailty through three clinical analysis tests – the Edmonton frail scale (EFS), the Montreal cognition assessment (MoCA-J), and the Timed Up and Go Test (TUG).
“Both the EFS and the MoCA-J gave us an indication of the individuals cognitive function, whereas the TUG allowed us to assess their motor ability,” said Professor Mitsuhiro Yanagida, who runs the Unit at OIST. “Between them, they also showed health status, mood, short-term memory and other indications, so they gave us a clear idea of who suffered from the disorder.”
By using these three tests, the researchers found that nine out of the 19 individuals fit into the category of being frail whereas the other ten did not, however some still did suffer from cognitive impairment or hypomobility, a syndrome which hinders movement.
Identifying markers in the blood
Next, the researchers took blood samples from the 19 patients and had a close look at the metabolites – small molecules of amino acids, sugars, nucleotides and more that make up our blood. They tested 131 metabolites and found that 22 of them correlated with frailty, cognitive impairment and hypomobility. Patients who suffered from these disorders tended to have lower levels of most of these metabolites.
“Blood metabolites are useful as biomarkers for finding, diagnosing and observing symptoms of frailty,” said Dr. Takayuki Teruya, Research Unit Technician in the G0 Cell Unit. “By using a simple blood test, we could start to diagnose frailty early on and lengthen healthy life expectancies by early intervention.”
The 22 metabolites identified included antioxidant metabolites, amino acids and muscle or nitrogen related metabolites. Fifteen of them correlated with frailty, six indicated cognitive impairment and twelve indicated hypomobility. The metabolites that correlated with frailty overlapped with five of those that indicated cognitive impairment and six that indicated hypomobility.
These metabolites include some of the aging markers in healthy people reported by the same group in 2016. This suggests that the severity of biological aging, which varies between individuals, could be monitored from an early stage of old age by measuring blood biomarkers.
“Notably, we found that levels of the antioxidant, ergothioneine, decreased in the frail patients,” said Professor Yanagida, “This metabolite is neuroprotective, meaning that people who suffer from frailty are more vulnerable to oxidative stress.”
The research indicates that frailty has a distinct metabolomic profile when compared to other age-related disorders. By demonstrating a link between these metabolites and the symptoms of the disorder, these findings could lead to a different approach to diagnosing and treating frailty.
The researchers in the G0 Cell Unit at OIST collaborated with Dr. Masahiro Kameda and Professor Hiroshi Kondoh in the Geriatric Unit, Graduate School of Medicine at Kyoto University. Kyoto University and OIST have jointly applied for a patent for these findings.
3.2 Aging markers in human urine: A comprehensive, non-targeted LC-MS study.
Urination is a primary route by which the body eliminates water-soluble waste products. Human urine contains diverse small molecules called metabolites, such as amino acids, sugars, and fatty acids, etc. Types of metabolites and their abundances are affected by health, disease, diet, and lifestyle, and have been studied for potential use as markers of health and disease. Urine has been broadly utilized for diagnosis of renal dysfunction in diverse kidney diseases. Nonetheless, because urinary metabolites originate in all organ systems, urinary metabolites may be useful to examine human aging in a synthetic way. Urinary metabolites are promising biological samples for monitoring health parameters if the metabolic processes resulting in production of those metabolites can be fully understood. In this study, published in FASEB BioAdvances, we reported metabolites related to human aging, which to date, few comprehensive, non-targeted studies have attempted to do.
We analyzed urinary metabolites in 13 young (30±3 yrs) and 14 (76±4 yrs) elderly subjects, using comprehensive metabolomics to identify metabolites linked to aging. Ninety-nine urinary metabolites were identified and quantified using liquid chromatography-mass spectrometry (LC-MS) and the peak identification software MZmine 2. These compounds were subdivided into 12 groups, containing standard amino acids, methylated amino acids, acetylated and other amino acids, nucleosides, nucleobases, and their derivatives, sugar derivatives, sugar phosphates, vitamins and coenzymes, choline and ethanolamine derivatives, carnitines, organic acids, and an antioxidant.
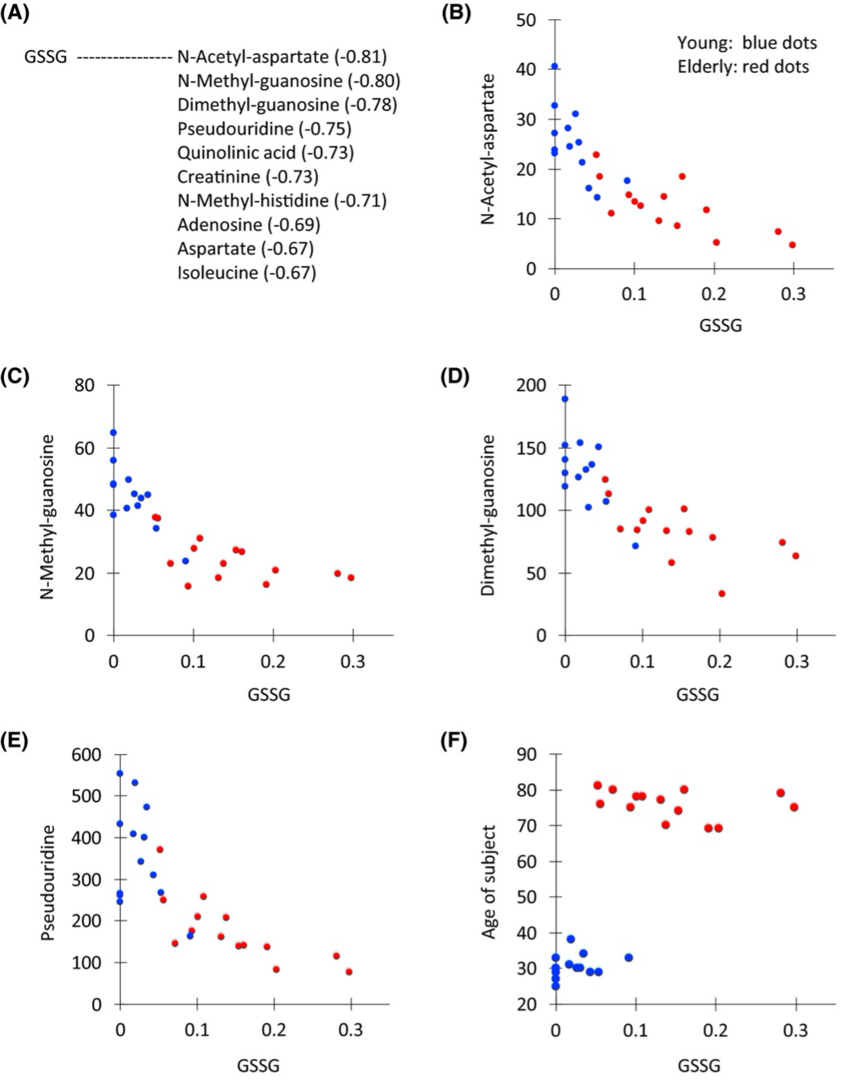
Quantitative analysis of 99 metabolites revealed 55 that displayed significant differences in abundance between the two groups. Forty-four did not show a statistically significant relationship with age. Interestingly, 55 metabolites declined in abundance in urine of elderly patients, whereas only two increased. One of the latter is oxidized glutathione, called glutathione disulfide (GSSG). The reduced form, glutathione, activates many molecules by reduction. In urine of young people, neither the reduced nor oxidized form was detected, while in urine of elderly patients, the oxidized form is abundant. The reason is probably due to the absence or declining effectiveness of a mechanism to metabolize and reprocess GSSG by the elderly. Such a reductive mechanism does exist in young subjects; however, the redox environment regarding glutathione appears to be altered during aging. Inability or declined efficiency to reduce oxidized GSSG at any recycling step may accelerate aging. The abundance of inactive glutathione disulfide in urine was inversely related to levels of pseudouridine, creatinine, and other metabolites, which were more abundant in young subjects.
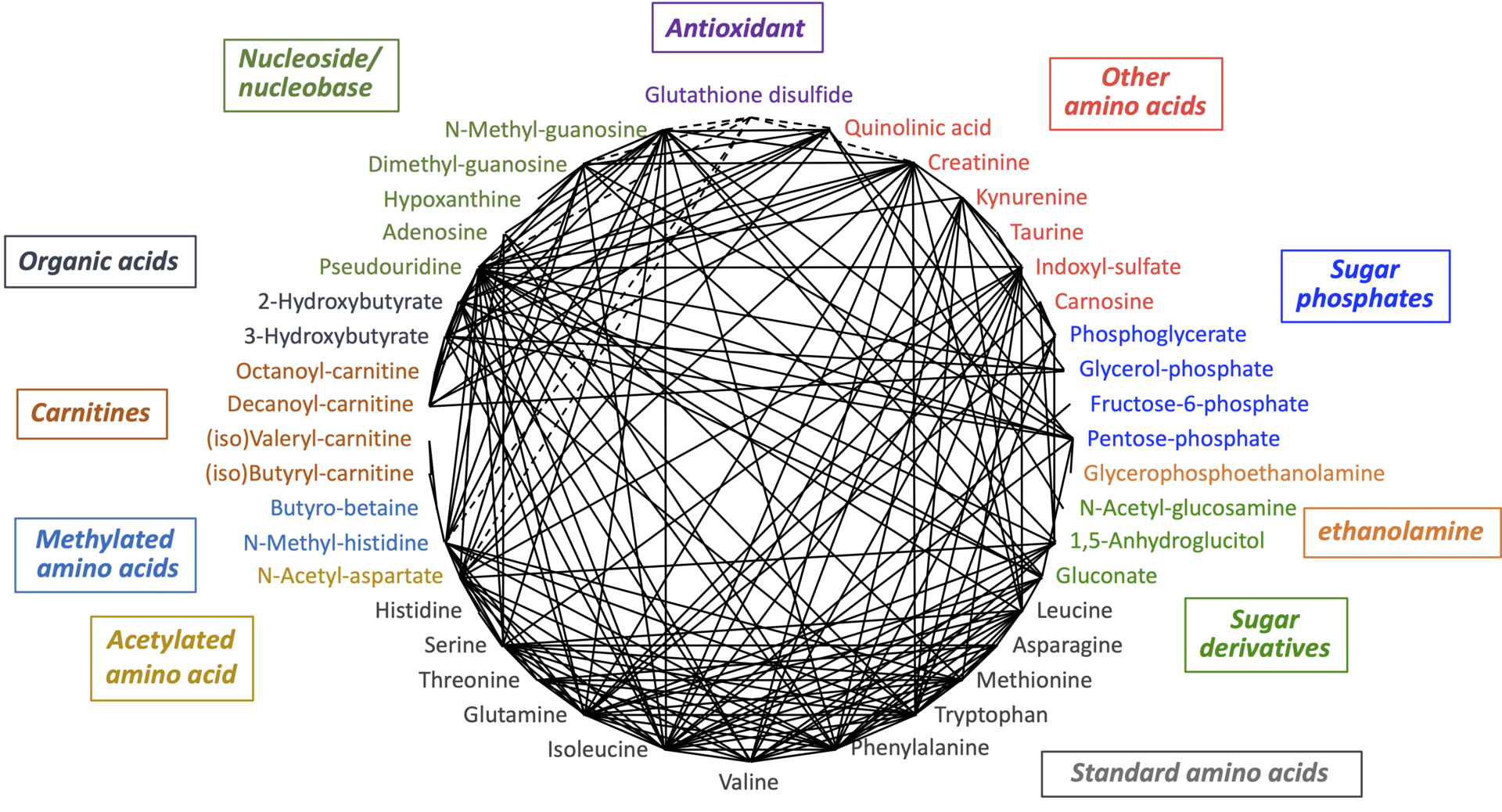
Declining 53 urinary metabolites in the elderly included standard amino acids, modified amino acids, sugar metabolites, carnitines, butyrates, purines/pyrimidines, choline/ethanolamine metabolites that have not previously been linked to aging. The majority of age-linked metabolites consisting of 42 including creatinine of 55 age-linked compounds formed a large positive/negative correlation network (correlation coefficients r>0.7).
Collectively, our data suggest that human aging affects the metabolite composition of urine much more extensively than previously thought, offering valuable non-invasive means of obtaining age-related information. Since urine age information is different from the information obtained from blood, these may reflect different aspects of aging and complementary to the information of aging state.
3.3 Multiple nutritional phenotypes of fission yeast mutants defective in genes encoding essential mitochondrial proteins
Mitochondria are essential for regulation of cellular respiration, energy production, small molecule metabolism, anti-oxidation and cell ageing, among other things. While the mitochondrial genome contains a small number of protein-coding genes, the great majority of mitochondrial proteins are encoded by chromosomal genes.
In humans, many diseases that impact brain and muscle functions are caused by malfunctioning mitochondria under increased oxidative stress, so full understanding of mitochondrial functions is important for human longevity.
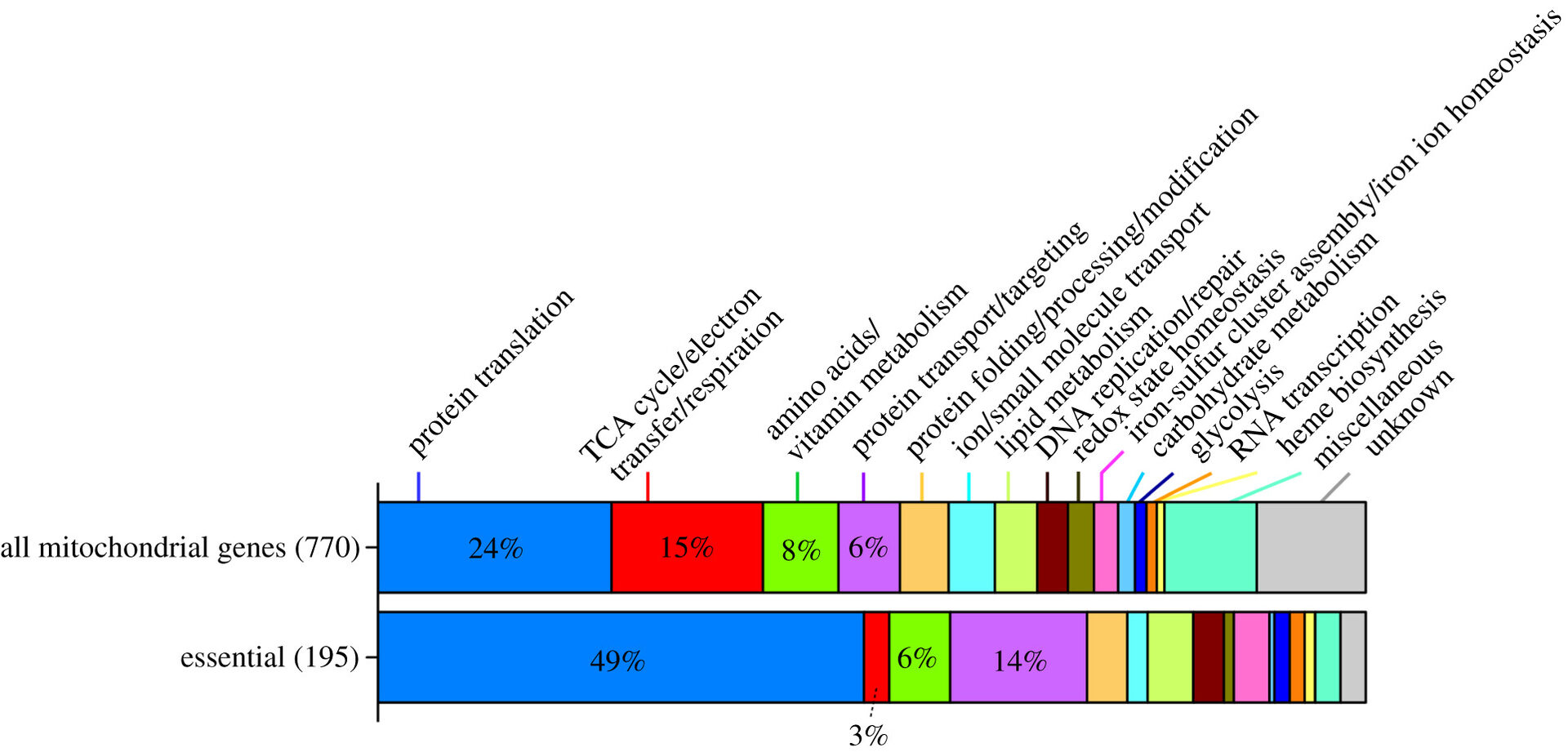
In the fission yeast Schizosaccharomyces pombe, 770 proteins encoded by chromosomal genes occur in mitochondria. Of these, 195 proteins, many of which are implicated in translation and transport, are absolutely essential for viability (Figure 4), and most of these mitochondrial proteins are conserved in mammals.
We isolated and characterized eight temperature-sensitive (ts) strains with mutations in essential mitochondrial proteins. These mutants are also sensitive to limited nutrition (glucose and/or nitrogen), resulting in low-glucose-sensitive and ‘super-housekeeping’ (shk) phenotypes (Figure 5). They fail to produce colonies under low-glucose conditions at the permissive temperature or lose cell viability under nitrogen starvation at the restrictive temperature. The majority of these ts mitochondrial mutations may cause defects of gene expression in the mitochondrial genome. mrp4 and mrp17 are defective in mitochondrial ribosomal proteins. ppr3 is defective in rRNA expression, and trz2 and vrs2 are defective in tRNA maturation.
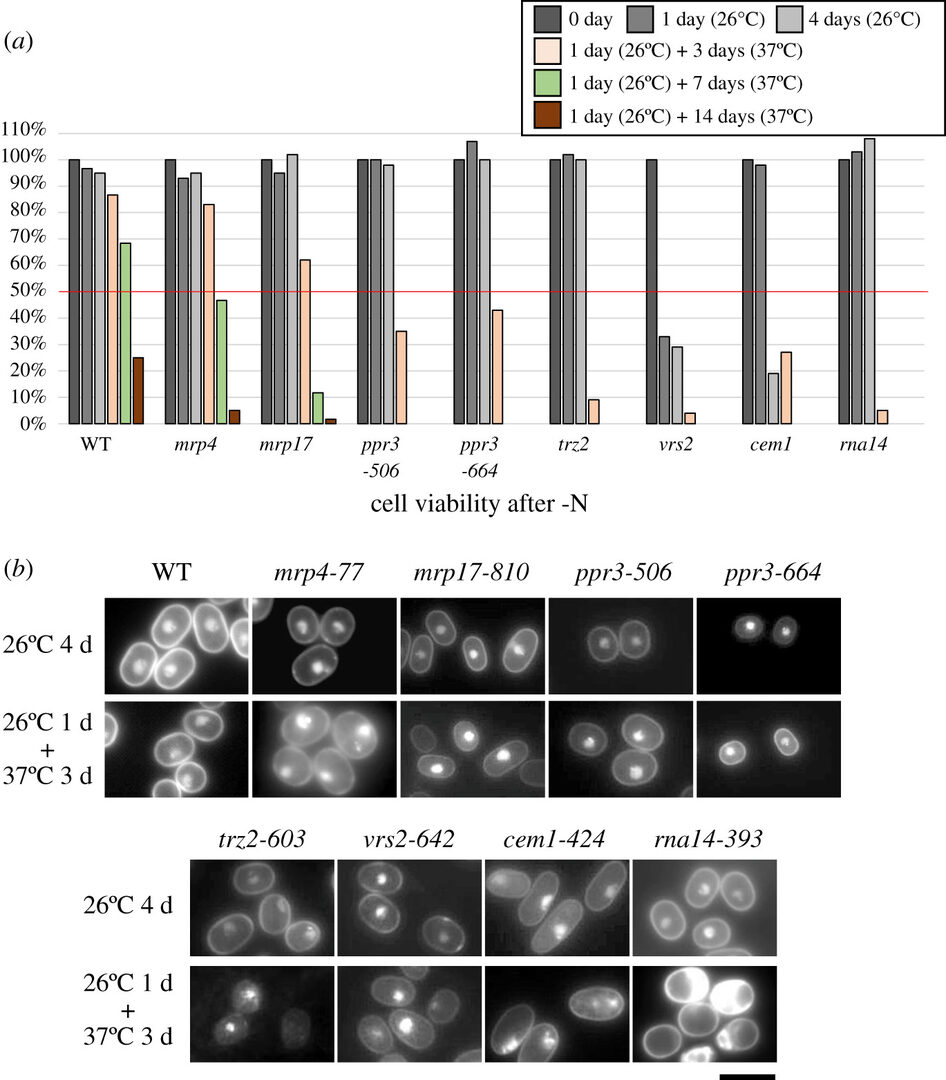
(b) Cellular and nuclear morphologies of the indicated ts mutant strains.
4. Publications
4.1 Journals
- Uehara L, Saitoh S, Mori A, Sajiki K, Toyoda U, Masuda F, Soejima S, Tahara Y, Yanagida M(2021)Multiple nutritional phenotypes of fission yeast mutants defective in genes encoding essential mitochondrial proteins. Open Biology [PubMed]
- Kameda M, Teruya T, Yanagida M, Kondoh H (2021) Reply to Pan et al.: Whole blood metabolome analysis combined with comprehensive frailty assessment. Proc Natl Acad Sci USA (PNAS) [PubMed]
- Kondoh H, Kameda M, Yanagida M (2020) Whole Blood Metabolomics in Aging Research. Int J Mol Sci [PubMed]
- Teruya T, Goga H, Yanagida M (2020) Aging markers in human urine: A comprehensive, non‐targeted LC‐MS study. FASEB BioAdvances [FASEB]
- Kondoh H, Teruya T, Yanagida M (2020) Metabolomics of human fasting: new insights about old questions. Open Biol [Royal Society Publishing]
- Kameda M, Teruya T, Yanagida M, Kondoh H (2020) Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proc Natl Acad Sci USA (PNAS) [PubMed]
4.2 Books and other one-time publications
Nothing to report
4.3 Oral and Poster Presentations
- Yanagida, M. Relationship between ergothioneine and human cognitive ability, 1St Ergo Meeting, Online, 8 Oct. (2020)
5. Intellectual Property Rights and Other Specific Achievements
- Yanagida M, Kondoh H, Kameda M, Teruya T. Blood metabolic markers for frail elderly patients. patent application in Japan, JP2020-168017 (filing date 02.10.2020)
- Yanagida M, Teruya T. Evaluation method for the risk of Alzheimer's disease using blood metabolites as an index. provisional patent application in Japan. JP2020-168919 (filing date 06.10.2020)
- Yanagida M, Teruya T. The use of saliva and urine metabolites as human-aging biomarkers. patent application in Japan. JP2020-559007 (filing date 22.10.2020)
6. Meetings and Events
Nothing to report
7. Other
Nothing to report.



