FY2013 Annual Report
Cellular and Molecular Synaptic Function Unit
Professor (Adjunct) Tomoyuki Takahashi
Research Theme: Regulatory mechanisms for transmitter release

Abstract
In presynaptic terminals, various molecular machineries are thought to operate for regulating neurotransmitter release, but, because of technical shortage, much remains to be studied for the molecular mechanism underlying presynaptic regulatory functions. To address this issue, we have previously established pre- and postsynaptic patch-clamp recordings at the mammalian central giant synapse calyx of Held visualized in thin slice. Using this method in combination with other techniques, we are currently studying endocytosis and trafficking mechanisms of synaptic vesicles and their influence on synaptic functional homeostasis. Regarding this issue, in FY 2013, we have clarified that a retrograde exo-endocytic balancing mechanism is mediated by Rho-kinase. Another important question we have been asking is the presynaptic molecular mechanisms underlying synaptic maturation. Regarding this question, we have discovered in this FY that activity-dependent neurotrophin-TrkB signaling induces developmental switch of presynaptic Ca2+ channel subtypes mediating neurotransmitter release. Through these studies, we aim at providing a novel insight into the homeostatic mechanism of brain function and the mechanism by which it is established through postnatal development.
Our progress in FY2013 is as follows:
(1) Rho-kinase accelerates synaptic vesicle endocytosis by linking PKG activity to PIP2 synthesis (Taoufiq Z, Eguchi K & Takahashi T, 2013 J Neurosci 33, 12099-12104)
(2) Activity-dependent neurotrophin signaling underlies developmental switch of Ca2+ channel subtypes mediating neurotransmitter release (Miki T, Hirai H & Takahashi T, 2013 J Neurosci 33, 18755-18763)
1. Staff
- Dr. Tomoyuki Takahashi, Professor
- Dr. Hiroshi Takagi, Group Leader
- Dr. Kohgaku Eguchi, Researcher
- Dr. Tetsuya Hori, Researcher
- Dr. Laurent Guillaud, Researcher
- Dr. Setsuko Nakanishi, Researcher
- Dr. Zacharie Taoufiq, Researcher
- Dr. Masashi Ohmachi, Researcher
- Dr. Dimitar Dimitrov, Specialist, Technician
- Ms. Lashmi Piriya Ananda Babu, OIST Ph.D. Student (from May, 2013)
- Mr. Yunyun He, Research Intern (from October, 2013 to January, 2014)
- Ms. Shivani Sathish, Research Intern (from January, 2014)
- Mr. Abdelmoneim Farid Abdelaziz Elsayed Eshra, Special Research Student (from February, 2014)
- Ms. Sayori Gordon, Research Administrator
2. Collaborations
-
Research theme: Regulatory mechanisms for transmitter release
- Type of partnership: Joint research
- Name of partner organization: Doshisha University Faculty of Life and Medical Sciences
- Name of principal researcher: Tomoyuki Takahashi
- Researchers:
- Naoto Saitoh, Doshisha University
- Takafumi Miki, Doshisha University
-
Research theme: Developmental changes in the presynaptic calcium transient profiles associated with transmitter release
- Type of partnership: Scientific Collaboration
- Name of partner organization: Pasteur Institute
- Name of principal researcher: David DiGregorio
- Researchers:
- David DiGregorio, Pasteur Institute
- Yukihiro Nakamura, Pasteur Institute
3. Activities and Findings
3.1 Rho-kinase accelerates synaptic vesicle endocytosis by linking cyclic GMP-dependent protein kinase activity to phosphatidylinositol-4,5-bisphosphate synthesis. Taoufiq Z, Eguchi K and Takahashi T (2013). J Neurosci 33, 12099-12104.
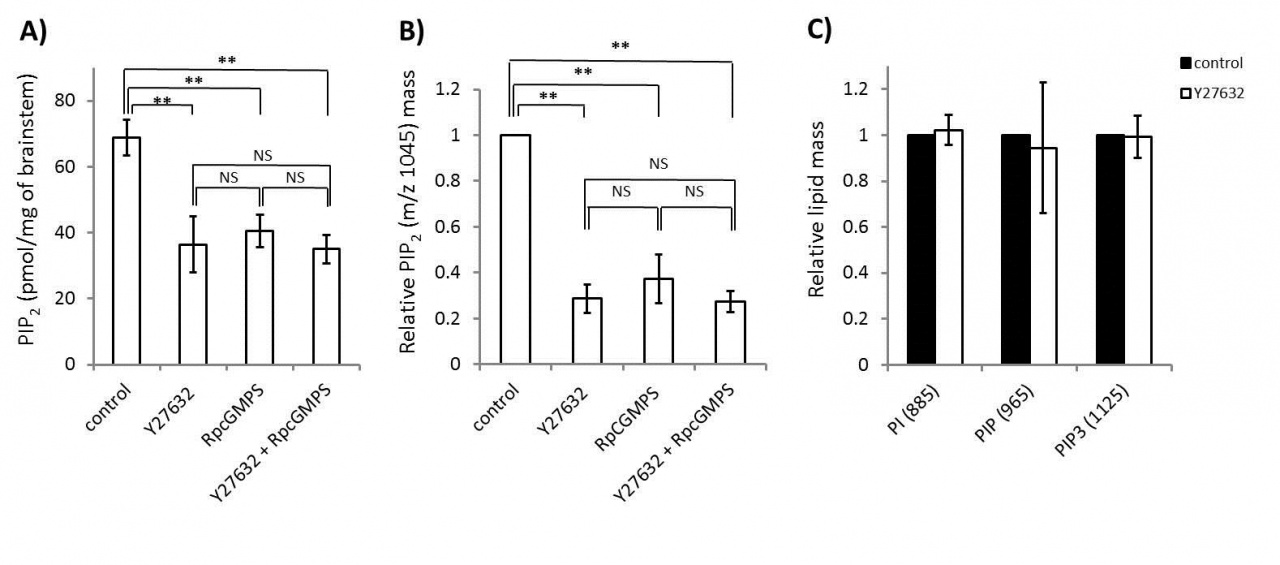
Rho-kinase plays diverse roles in cell motility. During neuronal development, Rho-kinase is involved in neuronal migration, and in neurite outgrowth and retraction. Rho-kinase remains highly expressed in mature neurons, but its physiological roles are poorly understood. We report that Rho-kinase plays a key role in the synaptic vesicle recycling system in presynaptic terminals. Vesicles consumed by excessive exocytosis are replenished by accelerating vesicle endocytosis via a retrograde feedback mechanism involving nitric oxide release from postsynaptic cells. This homeostatic control system involves presynaptic cyclic GMP-dependent protein kinase (PKG) and a plasma membrane phospholipid, phosphatidylinositol-4,5-bisphophate (PIP2) as we reported previously (Eguchi et al, 2012 Neuron). This time, we found that application of a Rho-kinase inhibitor (Y27632), a PKG inhibitor (RpcGMPs) or both together, reduced the PIP2 content in Wistar rat brainstem synaptosomes to a similar extent (Fig 1A ELISA; 1B, tandem mass spectrometry), whereas the Rho-kinase inhibitor had no effect on the level of the PIP2 precursor PI, PIP or the downstream product PIP3 (Fig 1C).
Fig 1
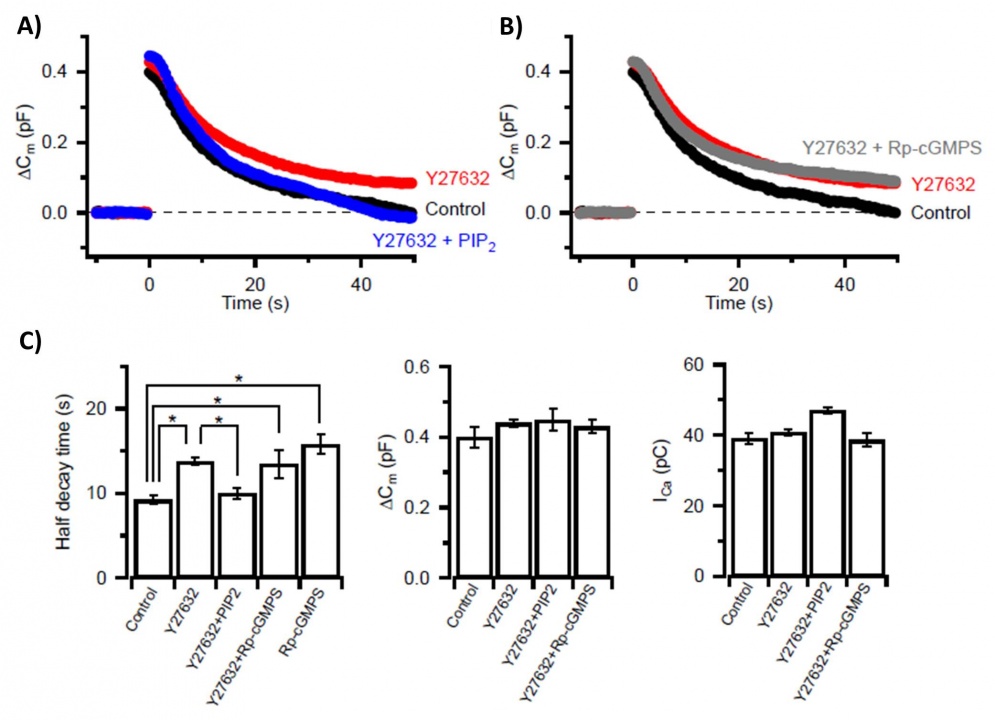
Likewise, application of the Rho-kinase inhibitor (Y27632) into the calyx of Held presynaptic terminal slowed vesicle endocytosis (Fig 2A, red trace) to the same degree as did application of the PKG inhibitor (RpcGMPs) (Fig 2C, Fig 4A, red). In intra-terminal co-application experiments, the endocytic slowing effect of the Rho-kinase inhibitor was occluded with the PKG inhibitor (Fig 2B, gray), and cancelled by PIP2 (Fig 2A, blue). These drugs had no effect on exocytic membrane capacitance change (Fig 2c, middle panel) or the amplitude of presynaptic Ca2+ currents triggering exocytosis (Fig 2c, right panel).
Fig 2
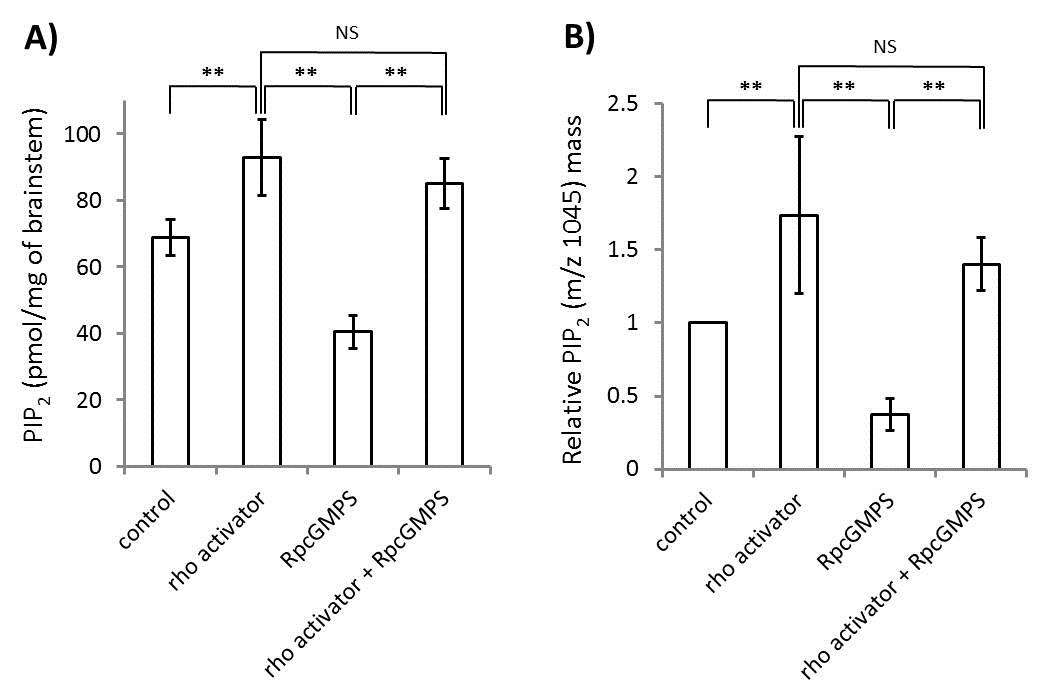
By contrast, a RhoA activator increased the PIP2 content and reversed the effect of the PKG inhibitor in brainstem synaptosomes (Fig 3A, ELISA; 3B, tandem mass spectrometry).
Fig 3
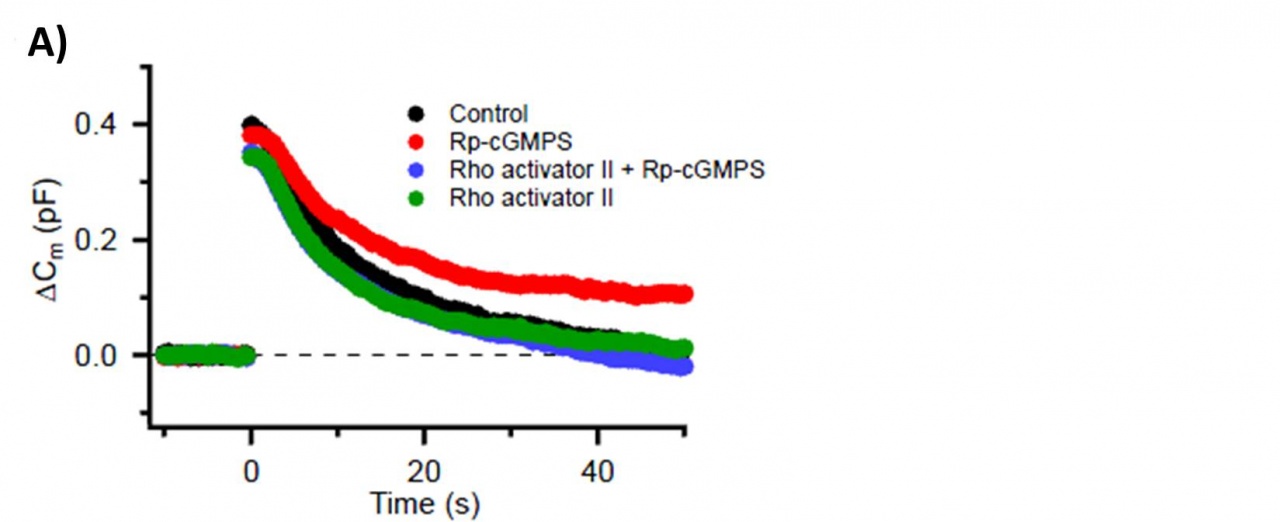
The RhoA activator, when loaded into calyceal terminals, also rescued the endocytic slowing effect of the PKG inhibitor (Fig 4A, blue). Furthermore, intra-terminal loading of anti-PIP2 antibody slowed vesicle endocytosis (Fig 4B, red) and blocked the rescuing effect of the RhoA activator (Fig 4B, blue).
Fig 4
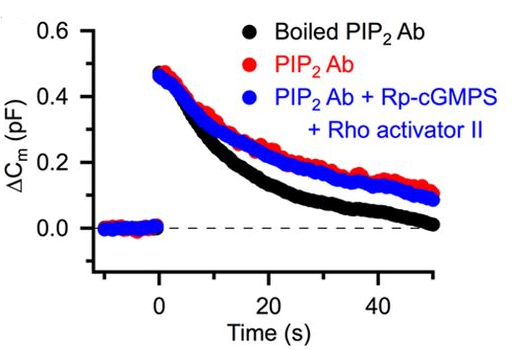
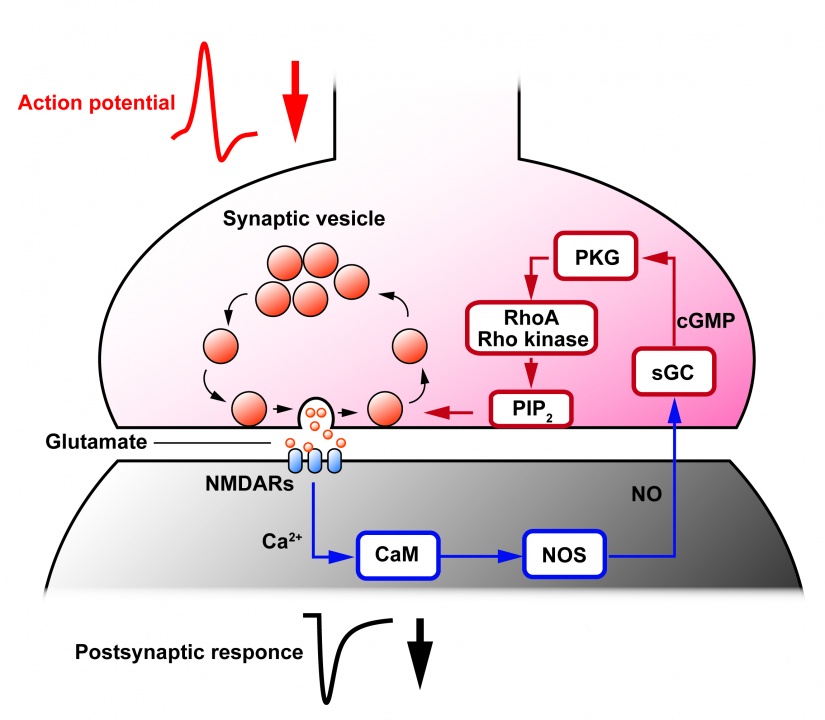
Together with our previous findings (Eguchi et al, 2012), we conclude that Rho-kinase links presynaptic PKG activity to PIP2 synthesis, thereby controlling the homeostatic balance of vesicle exocytosis and endocytosis in nerve terminals (Fig 5).
Fig 5
3.2 Activity-dependent neurotrophin signaling underlies developmental switch of Ca2+ channel subtypes mediating neurotransmitter release. Miki T, Hirai H, and Takahashi T(2013)J Neurosci 33, 18755-18763.
Presynaptic P/Q type, but not N type, Ca2+ channels undergo facilitations upon repetitive stimulation (Ishikawa et al, 2005). Using presynaptic action potential waveform voltage-clamp analysis, we previously determined that this mechanism accounts for 50% of paired-pulse facilitation with a 5 ms interval (Hori & Takahashi, 2009). Thus, this P/Q type Ca2+ channel facilitation is an important regulatory mechanism of transmitter release for strengthening the synaptic efficacy.
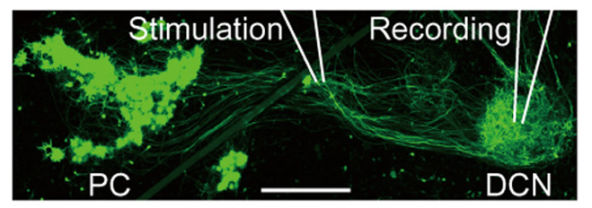
At many synapses, including the calyx of Held and cerebellar Purkinje-DCN synapses, both N and P/Q type Ca2+ channels mediate transmitter release, but 2-3 weeks after birth in rodents, N type Ca2+ channels are entirely replaced by P/Q type Ca2+ channels in these nerve terminals (Iwasaki et al, 2000), but not in their somata (Inchauspe et al, 2004). To address mechanisms underlying this Ca2+ channel switching, we developed cerebellar slice culture preparation, where IPSCs originating from EGFP-labeled Purkinje cells (PC) were recorded from neurons in the deep cerebellar nuclei (DCN, Fig 6).
Fig 6
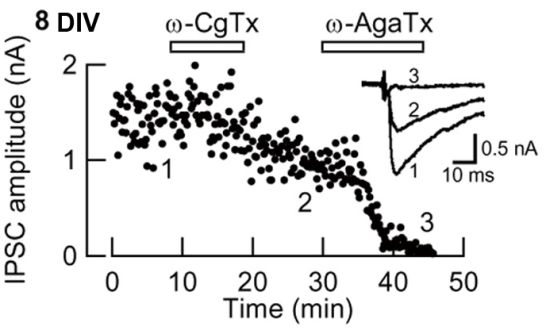
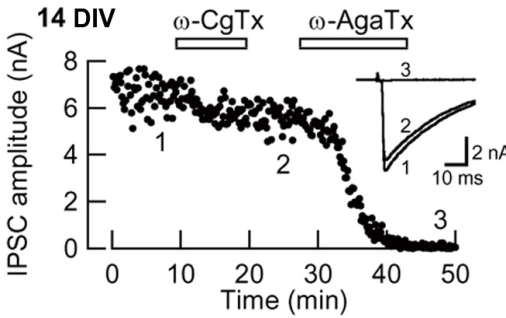
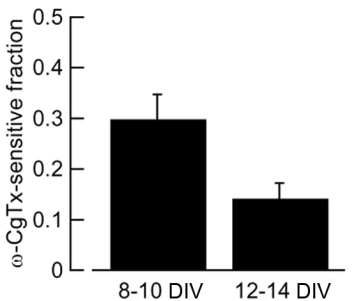
In this preparation, the developmental N-to-P/Q channel switch (Iwasaki et al, 2000) was replicated (Fig 7). Namely the w-conotoxin-sensitive fraction of IPSCs (N-type mediated) decreased and became largely w-Agatoxin-sensitive (P/Q-type mediated) at 12-14 day in vitro (DIV).
Fig 7
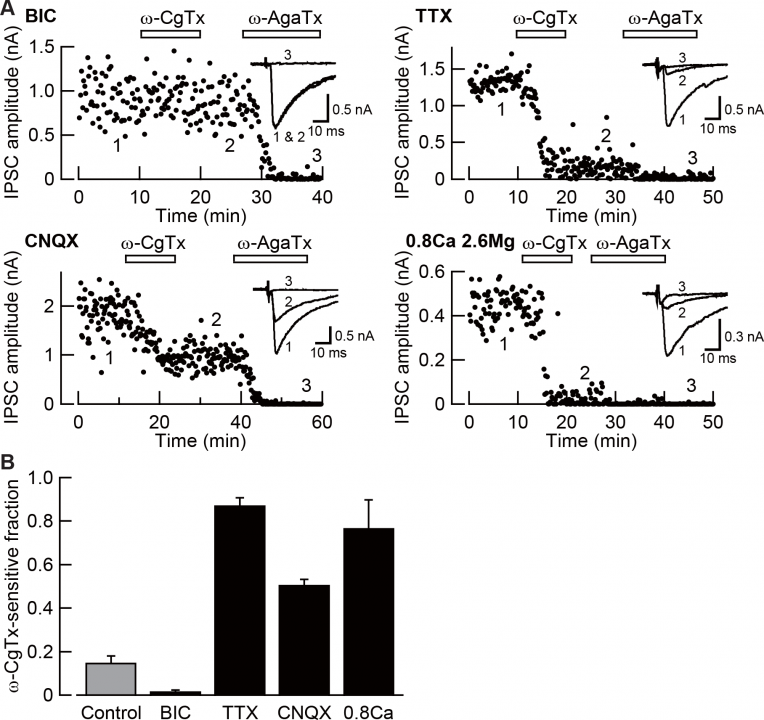
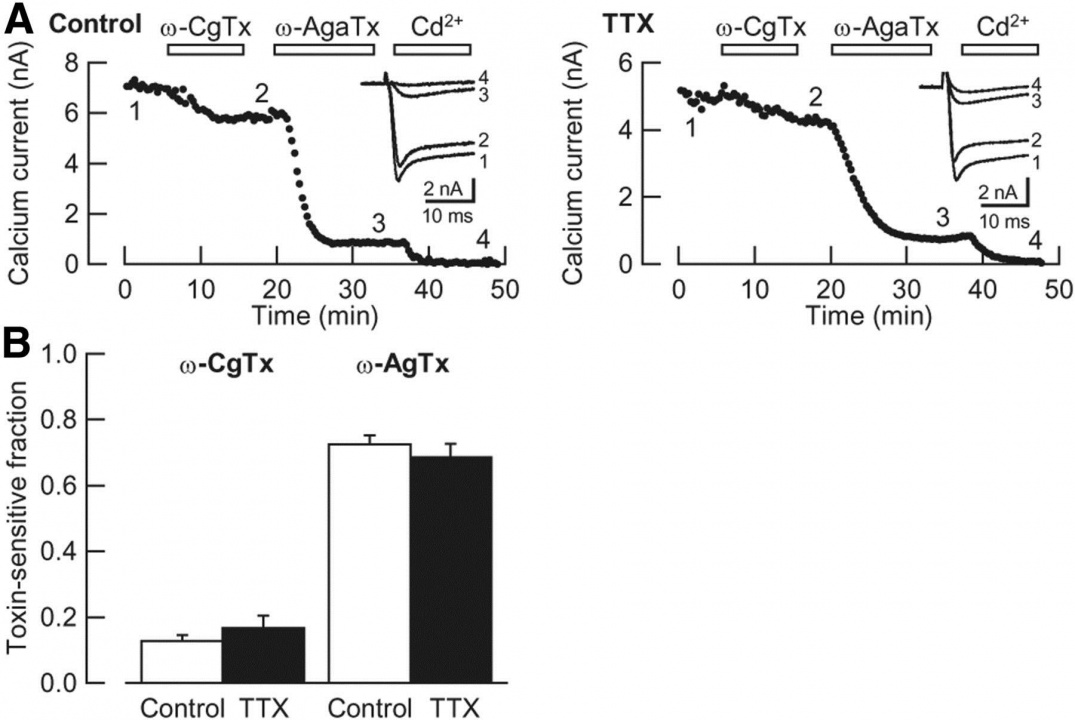
We found that neuronal activity is essential for this N-to-P/Q channel switch (Fig 8). Namely, enhanced neuronal activity, produced by blocking inhibitory synaptic transmission with chronically applied bicuculline (BIC), abolished N-type channel mediated (w-conotoxin-sensitive) fraction of IPSCs, whereas blocking excitatory synaptic transmission with CNQX, or blocking action potential generation with TTX, increased the N-type fraction. Reduction of [Ca2+] (from 2 mM to 0.8 mM) combined with an increase in [Mg2+](from 1 mM to 2.6 mM) also increased N-type channel fraction of IPSCs, suggesting that Ca2+ influx occurring under neuronal activity is essential for for the N-to-P/Q type Ca2+channel switch.
Fig 8
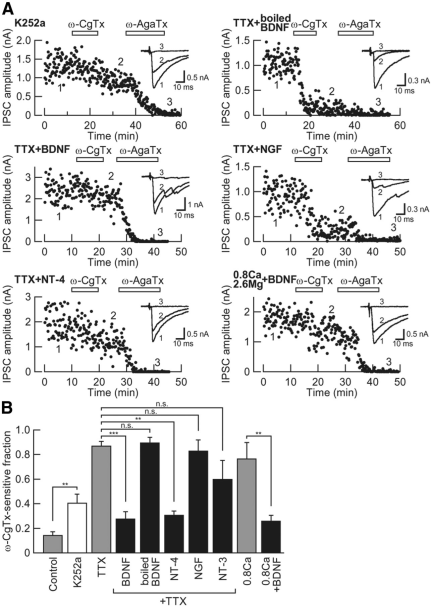
Neurotrophin signaling was operating downstream of the neuronal activity for switching Ca2+ channel subtypes, as indicate by the following results (Fig 9). First, the pan Trk blocker K252a mimicked the effect of TTX, increasing the N-type channel fraction of IPSCs. Second, the TrkB ligand BDNF or NT4 reversed the effect of TTX, whereas the TrkA ligand NGF or the TrkC ligand NT3 had no such effect. Third, BDNF reversed the up-regulatory effect of the low Ca2+/high Mg2+ culture media on the N-type channel fraction of IPSCs These results suggest that neuronal activity triggers Ca2+ dependent release of BDNF and/or NT-3 from cerebellar cells, thereby causing the N-to-P/Q type Ca2+ channel switch in the Purkinje cell axon terminals.
Fig 9
When TTX or K252a was infused chronically from postnatal day (P) 6 to P12-14 into pup cerebella using osmotic pump (Fig 10) the N-to-P/Q type switch was blocked (with increased w-conotoxin-sensitive IPSC fraction) confirming the results in slice culture.
Fig 10
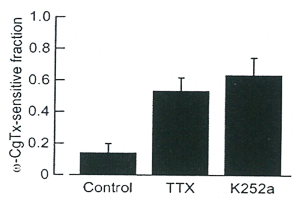
In PC somata in slice culture, in contrast to the nerve terminal, TTX had no effect on the composition of Ca2+ channel subtypes in (Fig 11), indicating that the activity dependent switch of Ca2+ channel subtypes occurs specifically in the nerve terminal.
Fig 11
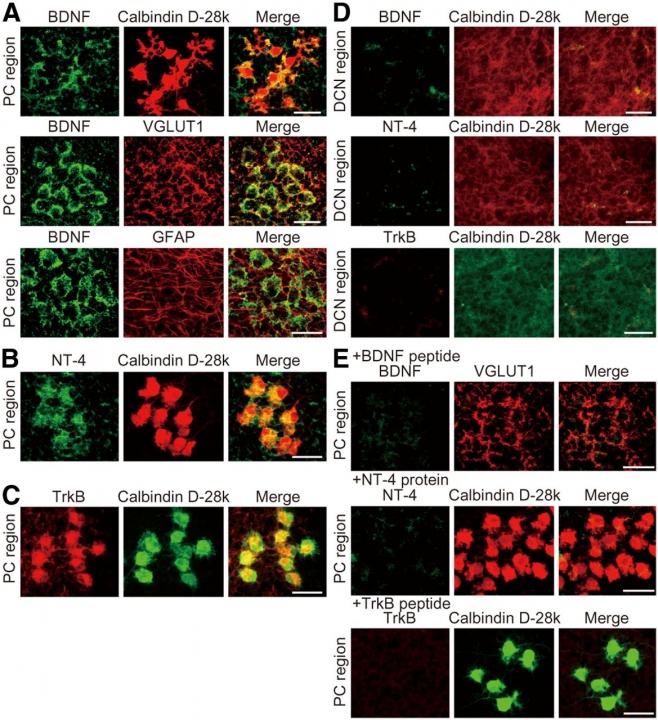
In the cerebellar cortex, BDNF was expressed on the VGLUT1-positive nerve terminals in the perisomatic PC region (Fig 12A), but not much in the DCN region (Fig 12D). GFAP-positive astrocytes in the PC region did not express BDNF (Fig 12A). NT-4 was expressed in PC somata, but not much in the DCN region (Fig 12B). Likewise, TrkB was expressed in PC somata, but not in the DCN region (Fig 12C).
Fig 12
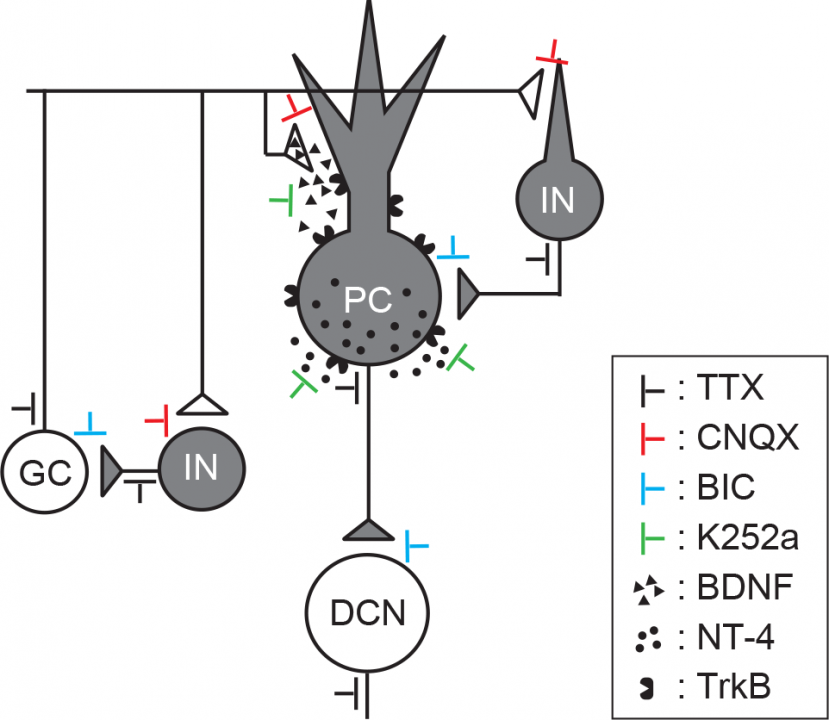
Our interpretations to present findings are as follows (Fig 13). Cerebellar neuronal activity induces release of BDNF from nerve terminals innervating PCs, such as parallel fiber terminals, and release of NT-4 from Purkinje cell somata. These neurotrophins activate TrkB on PC somata, thereby switching Ca2+ channel subtypes from N-type to P/Q type in PC axon terminals, but not in PC somata. K252a blocks these neurotrophin effects at PC somata. TTX blocks spike generation, thereby blocking the switch. CNQX blocks excitatory neurotransmission from granule cells (GC), thereby reducing neuronal activity of PCs. Bicuculline blocks inhibitory neurotransmission from interneurons (IN) to GC or PC, thereby enhancing activity of GCs and PCs.
Fig 13
Thus, during postnatal development, neuronal activity activates the neurotrophin-TrkB signaling pathway, thereby causing the N-to-P/Q channel switch in presynaptic terminal. Since this switch is restricted to the terminal, as yet unidentified Ca2+ channel type-specific binding proteins in the nerve terminal might mediate this developmental sorting mechanism.
4. Publications
4.1 Journals
Taoufiq, Z., Eguchi, K., Takahashi, T. "Rho-kinase accelerates synaptic vesicle endocytosis by linking cyclic GMP-dependent protein kinase activity to phosphatidylinositol-4,5-bisphosphate synthesis. " The Journal of Neuroscience 33(29): 12099-12104. (2013)
Miki, T., Hirai, H., Takahashi, T. "Activity-dependent neurotrophin signaling underlies developmental switch of Ca2+ channel subtypes mediating neurotransmitter release. " The Journal of Neuroscience 33(48): 18755-18763. (2013)
4.2 Books and Other One-Time Publications
Nothing to report
4.3 Oral and Poster Presentations
Oral Presentation
Eguchi, K. The retrograde regulation of synaptic vesicle endocytosis via RhoA/Rho-kinase at calyx of Held synapse. NIPS meeting, “Newly molecules for signal transduction and its physiological potentialities". National Institute for Physiological Sciences (NIPS), Okazaki, Aichi, Japan, September 26-27, 2013
Eguchi, K. The acceleration mechanisms of synaptic vesicle endocytosis by Rho-associated protein kinase.
2013 JSPS Core-to-Core Program Symposium “Mechanisms of Synaptic Transmission”,
Doshisha University, Kyoto, Japan, December 5-6, 2013
Hori, T. The physiological importance of synaptic vesicle filling rate with neurotransmitter.
2013 JSPS Core-to-Core Program Symposium “Mechanisms of Synaptic Transmission”,
Doshisha University, Kyoto, Japan, December 5-6, 2013
Hori, T. Physiological importance of the rate of synaptic vesicle filling for maintaining fast synaptic transmission in the mammalian CNS. The 91th Annual Meeting of the Physiological Society of Japan, Kagoshima University Korimoto Campus, Kagoshima, Japan, March 16-18, 2014
Poster presentation
Hori, T., Akahane, M., Takahashi, T. Estimation of the recycling pool size at the calyx of Held presynaptic terminal. Neuro 2013, Joint meeting of the 36th Annual Meeting of the Japan Neuroscience Society, the 56th Annual Meeting of the Japanese Society for Neurochemistry, and the 23rd Annual Conference of the Japanese Neural Network Society.Kyoto International Conference Center, Kyoto, Japan, June 20-23, 2013
Dimitrov, D., Takagi, H., Guillaud L., Nakanishi, S., Saitoh, N., Takahashi, T. The neurotrophin-3 receptor TrkC mediates structural and functional development of calyceal giant synapses in dissociated culture of auditory brainstem neurons. Neuro 2013, Joint meeting of the 36th Annual Meeting of the Japan Neuroscience Society, the 56th Annual Meeting of the Japanese Society for Neurochemistry, and the 23rd Annual Conference of the Japanese Neural Network Society.
Kyoto International Conference Center, Kyoto, Japan, June 20-23, 2013
Nakanishi, S. and Takahashi, T.
P/Q-type Ca2+ channels visualized with biotinylated &omega-Aga IV A toxin in transmission electron microscopy at the calyx of Held synapse. Neuro 2013, Joint meeting of the 36th Annual Meeting of the Japan Neuroscience Society, the 56th Annual Meeting of the Japanese Society for Neurochemistry, and the 23rd Annual Conference of the Japanese Neural Network Society. Kyoto International Conference Center, Kyoto, Japan, June 20-23, 2013
Taoufiq, Z., Eguchi, K., Takahashi, T. Rho-kinase accelerates synaptic vesicle endocytosis by linking PKG activity to phosphatidylinositol-4, 5-bisphophate synthesis. Neuro 2013, Joint meeting of the 36th Annual Meeting of the Japan Neuroscience Society, the 56th Annual Meeting of the Japanese Society for Neurochemistry, and the 23rd Annual Conference of the Japanese Neural Network Society. Kyoto International Conference Center, Kyoto, Japan, June 20-23, 2013
Ohyama, C., Hori, T., Eguchi, K., Takahashi, T. Fidelity of synaptic transmission depends upon presynaptic chloride concentrations. Neuro 2013, Joint meeting of the 36th Annual Meeting of the Japan Neuroscience Society, the 56th Annual Meeting of the Japanese Society for Neurochemistry, and the 23rd Annual Conference of the Japanese Neural Network Society. Kyoto International Conference Center, Kyoto, Japan, June 20-23, 2013
Guillaud, L., Dimitrov, D., Takahashi, T. Wnt7a and nicotine enhance synaptogenesis and vesicular glutamate transporter heterogeneity in cultured calyceal synapses. Society for Neuroscience 2013 (SFN). San-Diego, U.S.A., November 9-13, 2013
Taoufiq, Z.,Eguchi, K., Takahashi, T. Rho-kinase plays a key role in balancing exocytosis and endocytosis of synaptic vesicles via regulating the level of phosphatidylinositol-4,5-bisphosphate. Society for Neuroscience 2013 (SFN). San-Diego, U.S.A., November 9-13, 2013
5. Intellectual Property Rights and Other Specific Achievements
5.1 Grant:
Kohgaku Eguchi: Grant-in-Aid for Young Scientists B, KAKENHI (2012-2015).
6. Meetings and Events
6.1 Molecular and cellular imaging. 22th MPC Hamamatsu Practical course of Medical photonics,
including Ca imaging and TIRF observation at Hamamatsu University School of Medicine.
- Date: August 28-30, 2013
- Venue: Hamamatsu University School of Medicine, Hamamatsu, Shizuoka.
- Speaker: Hiroshi Takagi
6.2 Challenge of synaptologists into understanding of the structure and function of the neural
network.
- Venue: The 91th Annual Meeting of the Physiological Society of Japan,
- Dates: March 16, 2014
- Venue: Kagoshima University, Korimoto Campus, Kagoshima, Japan
- Organizer: Tetsuya Hori (OIST), Kouichi Hashimoto, Hiroshima University
7. Others
7.1 Patent
Dimitrov, D., Guillaud, L., Taoufiq. Z., Takahashi, T., NEURONAL CULTURE MEDIUM AND METHOD FOR PRODUCING IN VIVO-LIKE AND ENHANCED SYNAPTOGENESIS NEURON MODEL., Issuing Organization by WIPO. Patent# WO/2013/144999, October 3, 2013



