FY2018 Annual Report
Abstract
Hydrogels are soft materials with distinct three-dimensional (3D) networks capturing large water content. The similarity of hydrogels to natural tissue has made them the most promising biomaterials for use in human bodies. During the past several decades, hydrogels have attracted considerable attention from scientists in different research fields. With their contributions, the research in this field has experienced significant advances both in content and in style. Organometallic hydrogels, one special category of hydrogels in which metal ions participate in the 3D network, have become one of the pioneering types of soft materials. Endowed with the unique properties of metal ions and coordination chemistry’s structural diversity, this type of material has shown great potential for intelligent materials as part of a smart future.
1. Staff
- Dr. Guanying Li, Postdoc fellow
- Dr. Dingze Mang, Postdoc fellow
- Dr. Yujie Zhou, Postdoc fellow
- Dr. Xia Wu, Postdoc fellow
- Shijin Zhang, Graduate Student
- Sona Rani Roy, Graduate Student
- Xunwu Hu, Graduate Student
- Sachie Yukawa, Graduate Student
- Hitomi Shinzato, Administrative Assistant
2. Collaborations
2.1 Regulation of Hippo-signaling via lipid-raft-targeted molecular self-assembly
-
Description: Hippo signaling pathway controls multiple cellular functions that are central to tumorigenesis. Its importance in cancer cell proliferation, metastasis and cancer stem cell biology has been well recognized. Therefore, Hippo signaling components are considered as the most promising targets for cancer therapy. Such opportunities also present important challenges, including the identification of druggable components of the pathway, and drug development for signaling control.The objective of this proposal is to design and screen out functional synthetic peptides as upstream regulators of Hippo pathway for anticancer drug development. As shown in the schematic demonstration on the right, well-designed synthetic peptides will selectively bind/interact with cancer biomarkers (integrin, alkaline phosphatase, etc.) localized on lipid raft. The induced localized accumulation of peptides will initiate molecular assembly into nanostructures adhering to lipid rafts. The formation of nano-biointerface will regulate the spatial organization of lipid raft on plasma membrane. Through cytoskeleton, the activation of Hippo signaling will deactivate the core oncogene YAP repress cancer development.
- Type of collaboration: Joint research
- Researchers:
- Professor Z. C. Zhou, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences, China
- Prof. S. Jiao, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences, China
3. Activities and Findings
3.1 Co-assembly for higher order structures
The extracellular matrix (ECM) is the natural fibrous scaffold that regulates cell behavior in a hierarchical manner. By mimicking the dynamic and reciprocal interactions between ECM and cells, higher‐order molecular self‐assembly (SA), mediated through the dynamic growth of scaffold‐like nanostructures assembled by different molecular components, was developed. Designed and synthesized were two self‐sorted coumarin‐based gelators, a peptide molecule and a benzoate molecule, which self‐assemble into nanofibers and nanobelts, respectively, with different dynamic profiles. Upon the dynamic growth of the fibrous scaffold assembled from peptide gelators, nanobelts assembled from benzoate gelators transform into a layer‐by‐layer nanosheet, reaching ninefold increase in height. By using light and an enzyme, the spatial–temporal growth of the scaffold can be modified, leading to in situ height regulation of the higher‐order architecture.
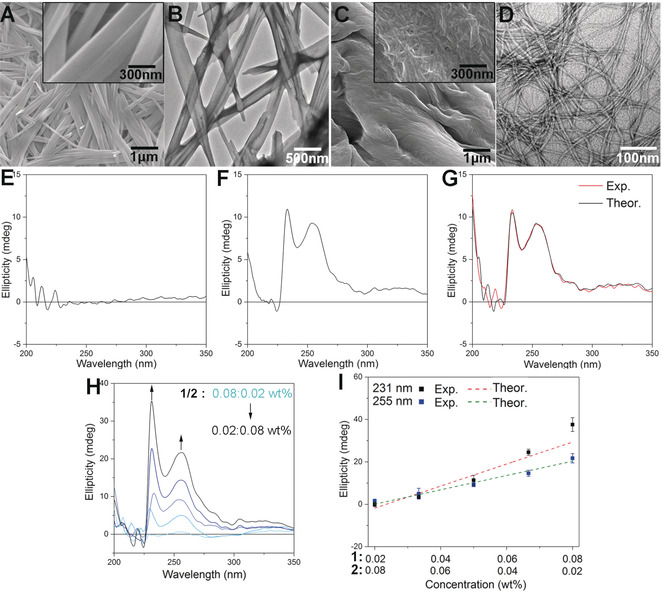
Figure 1: A) SEM and B) TEM images of SA of 1 in H2O/DMSO (v/v=9:1) at concentration of 0.8 wt %. C) SEM and D) TEM images of SA of 2 in H2O/DMSO (v/v=9:1) at concentration of 0.8 wt %. E)–G) CD spectra of E) 1 (0.05 wt %), F) 2 (0.05 wt %), and G) a 1/2 mixture ([1]=0.05 wt %, [2]=0.05 wt %) in H2O/DMSO (v/v=9:1). Exp. and Theor. represent experimental and theoretical CD spectra, respectively. H) CD spectra of a 1/2 mixture at various ratios in H2O/DMSO (v/v=9:1). I) Plots of CD intensities of the 1/2 mixture at 231 nm and 255 nm versus mixing ratios from H. Theoretical lines were calculated from the CD intensities of each component at various concentrations. Data represent mean±standard deviation of the mean (n=3).
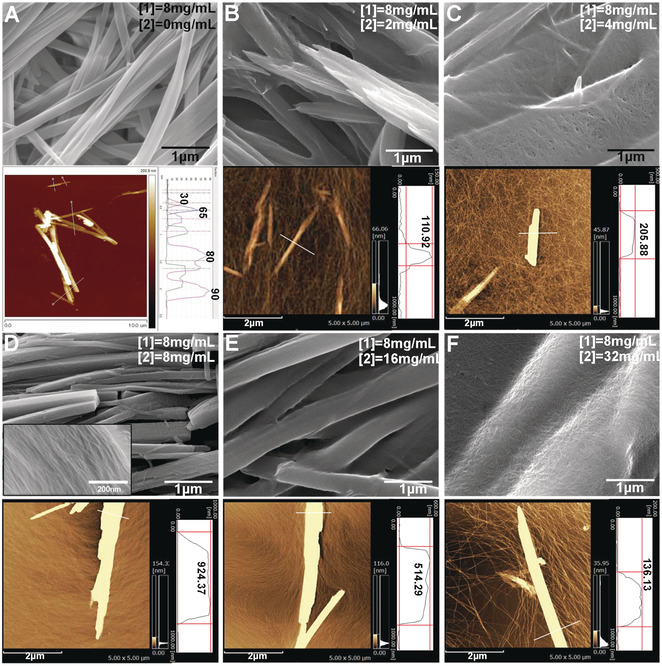
Figure 2: SEM images and the correlated AFM images with height profiles of A) SCSA of 1and B)–F) a mixture of 1 (8 mg mL−1) with 2 at various concentrations, from B) 2 mg mL−1, C) 4 mg mL−1, D) 8 mg mL−1, E) 16 mg mL−1, to F) 32 mg mL−1 in H2O/DMSO (v/v=9:1). The inset SEM image in (D) is the section structure of layer‐by‐layer nanosheet.
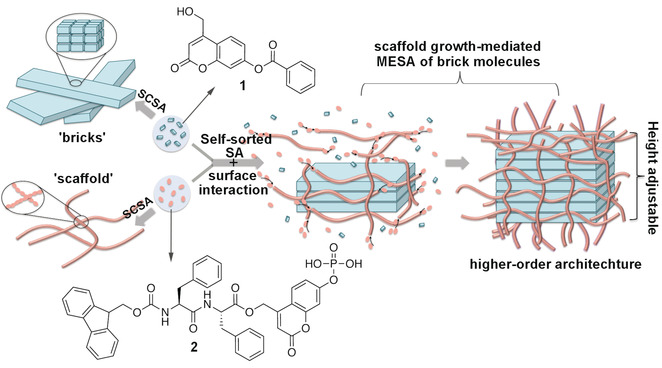
Figure 3: Illustration of higher‐order organization through the synergy of two types of self‐sorted assembly with molecular structures of gelators 1 and 2.
3.2 Plasma membrane insertion for anticancer study
Inspired by the metamorphosis of pore-forming toxins from soluble inactive monomers to cytolytic transmembrane assemblies, we developed self-assembly-directed membrane insertion of synthetic analogues for permeability alteration. An expanded π-conjugation-based molecular precursor with an extremely high rigidity and a long hydrophobic length that is comparable to the hydrophobic width of plasma membrane was synthesized for membrane-inserted self-assembly. Guided by the cancer biomarker expression in vitro, the soluble precursors transform into hydrophobic monomers froming assemblies inserted into the fluid phase of the membrane exclusively. Membrane insertion of rigid synthetic analogues destroys the selective permeability of the plasma membrane gradually. It wventually leads to cancer cell death, including drug resistant cancer cells.
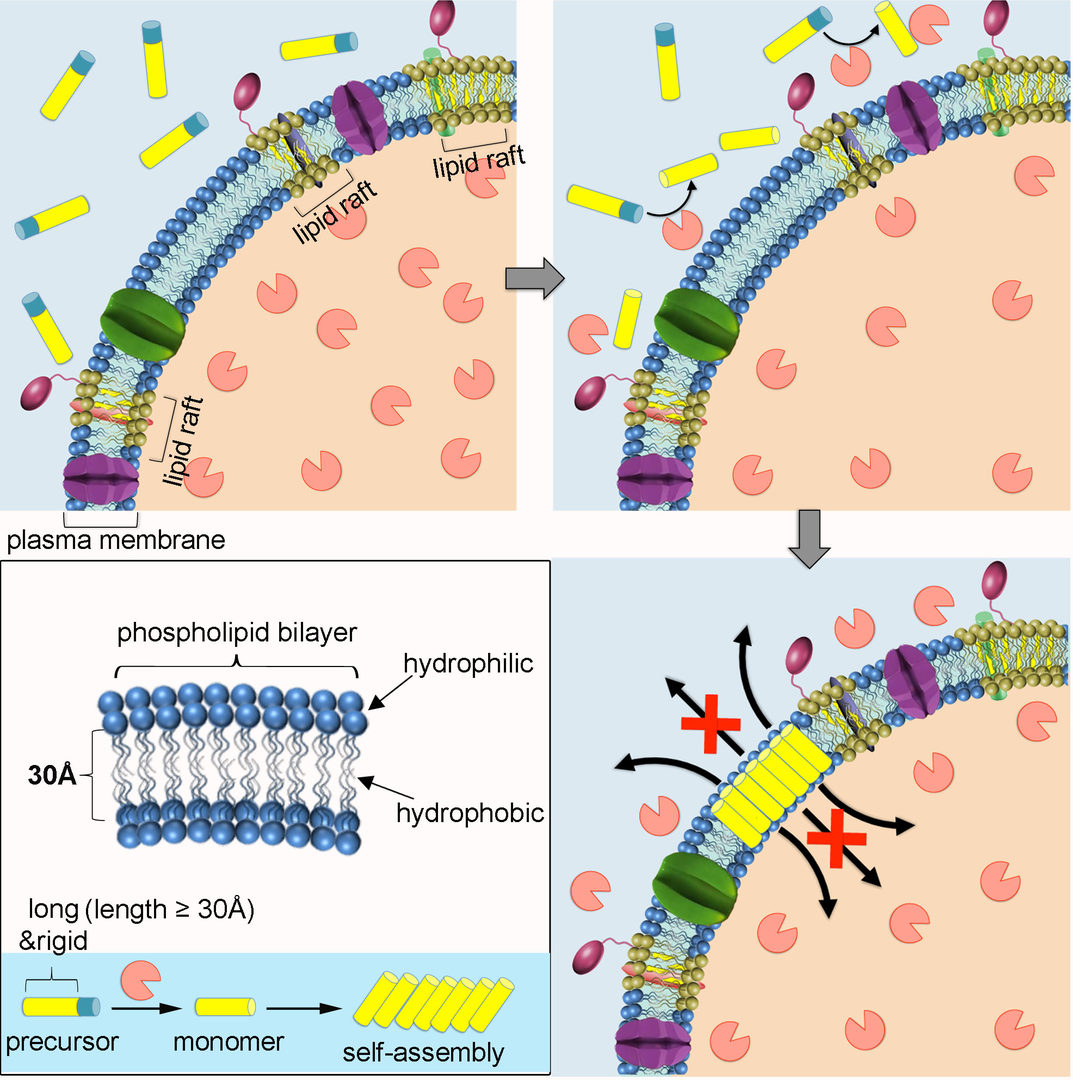
Figure 1: Schematic illustration of self-assembly-directed membrane insertion of a long and rigid synthetic analogue causing permeability alteration.
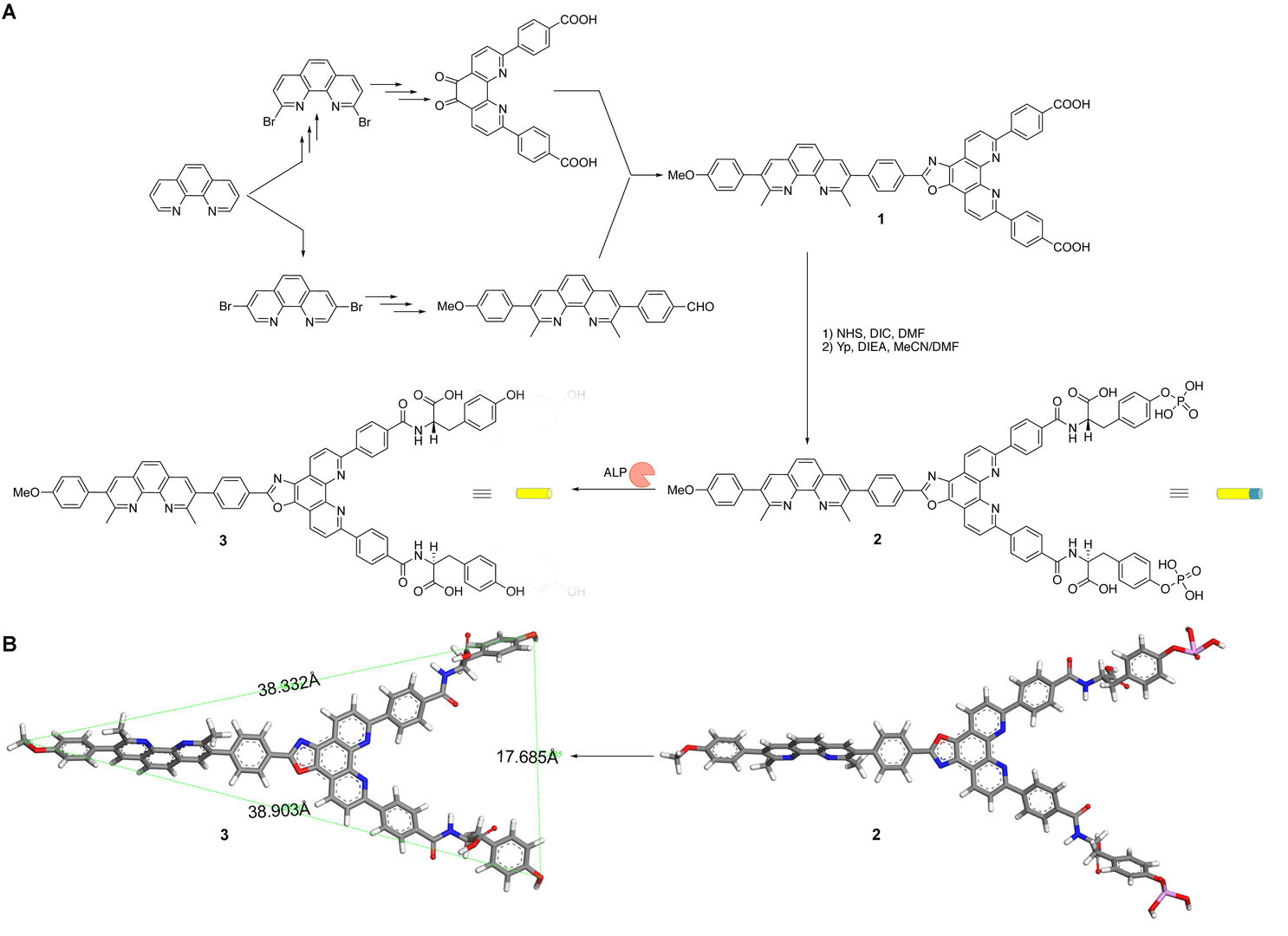
Scheme 1: (A) Synthetic path of precursor 2 with the enzyme-triggered transformation of 2 into the monomer of self-assembly 3 and (B) the stick models of geometrical energy-minimized structures of 2 and 3.
4. Publications
4.1 Journals
- Enming Du, Xunwu Hu, Guanying Li, Shijin Zhang, Dingze Mang, Sona Roy, Toshio Sasaki, and Ye Zhang.* "Self-assembly-Directed Cancer Cell Membrane Insertion of Synthetic Ananlogues for Permeability Alteration" Langmuir, 2019, 35, 7376 - 7382.
- Wei Ji, Shijin Zhang, Sachie Yukawa, Shogo Onomura, Toshio Sasaki, Kun'ichi Miyazawa, and Ye Zhang.* "Regulate Higher-order Organization through the Synergy of Two Self-sorted Assemblies" Angew. Chem. Int. Ed. 2018, 57, 3636-3640
4.2 Oral and Poster Presentations
- Ye Zhang, "Regulate Cancer Cell Mechanics by Synthetic Molecular Self-assembly", Oral presentation, International Conference of Molecular Engineering of Polymers (MEP-2018), Fudan University, Shanghai, China.
- Dingze Mang, Ye Zhang. "Enzyme-sorted-assembly of chiral peptides kills cancer cell via multi-subcellular targeting", Poster presentation, 10th International Peptide Symposium (55th National peptide symposium of Japan), Tokyo, Japan. Travel Award, ACIE/ChemBioChem Award.
- Shijin Zhang, Ye Zhang, "Esterase triggered disassembly of coumarin derivatives leading to fluorescent response", Poster presentation, 10th International Peptide Symposium (55th National peptide symposium of Japan), Tokyo, Japan.
- Sona Rani Roy, Ye Zhang, "Control of the Tumorigenic Signaling Pathways via Assembly of Integrin-targeted Synthetic Peptides", Poster presentation, 10th International Peptide Symposium (55th National peptide symposium of Japan), Tokyo, Japan.
- Sachie Yukawa, Ye Zhang, "Regulate Higher-order organization through molecular co-assembly"Poster presentation, 10th International Peptide Symposium (55th National peptide symposium of Japan), Tokyo, Japan. Travel Award.
- Ye Zhang, "Regulate Cancer Cell Mechanics by Synthetic Molecular Self-assembly", Invited oral presentation, OISTxKAIST joint symposium, OIST, Japan.
- Ye Zhang, "Regulate Higher-order Organization through the Syngery of Two Self-sorted Assembly", Oral Presentation, 69th Annual Meeting of Division of Colloid and Surface Chemistry, Chemistry Society of Japan, Tsukuba, Japan.
- Ye Zhang, "Infiltrate Ovarian Cancer with Raft-targeted Assemblies Overcoming Tumor Heterogeneity", Oral Presentation, The 12th SPSJ (the Society of polymer science Japan) International Polymer Conference, Hiroshima, Japan.
- Ye Zhang, 'Regulate Hippo signaling via lipid-raft-targeted molecular assembly for ovarian cancer treatment', invited oral presentation, The SPIRITS International Symposium, Kyoto University, Japan.
5. Intellectual Property Rights and Other Specific Achievements
Nothing to report
6. Meetings and Events
6.1 Seminar Title in Full
- Date: June 17, 2010
- Venue: OIST Campus Lab1
- Speaker: Dr. First Last (University of Something)
6.2 Something Group for Something on Something (SG2S)
- Date: June 17, 2010
- Venue: OIST Campus Lab1
- Co-organizers: The Institute of All But Cats (IABC)
- Speakers:
- Dr. First Last (Affiliation)
- Dr. First Last (Affiliation)
- Dr. First Last (Affiliation)
7. Other
Nothing to report.



